Genetic analysis of the RcsC sensor kinase from Escherichia coli K-12
- PMID: 11807084
- PMCID: PMC134787
- DOI: 10.1128/jb.184.4.1204-1208.2002
Genetic analysis of the RcsC sensor kinase from Escherichia coli K-12
Abstract
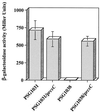
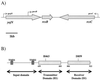
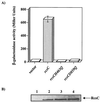
The Rcs two-component pathway is involved in the regulation of capsule production in Escherichia coli. RcsC is predicted to be the sensor component of this two-component pathway, and in this study we present the first genetic data that support the role of RcsC as a hybrid sensor kinase.
Figures
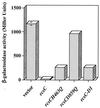
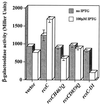
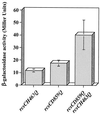
References
-
- Bassler, B. L., M. Wright, R. E. Showalter, and M. R. Silverman. 1993. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9:773-786. - PubMed
-
- Clarke, D. J., I. B. Holland, and A. Jacq. 1997. Point mutations in the transmembrane domain of DjlA, a membrane-linked DnaJ-like protein, abolish its function in promoting colanic acid production via the Rcs signal transduction pathway. Mol. Microbiol. 25:933-944. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

