Localization of the cortex lytic enzyme CwlJ in spores of Bacillus subtilis
- PMID: 11807087
- PMCID: PMC134822
- DOI: 10.1128/jb.184.4.1219-1224.2002
Localization of the cortex lytic enzyme CwlJ in spores of Bacillus subtilis
Abstract
The enzyme CwlJ is involved in the depolymerization of cortex peptidoglycan during germination of spores of Bacillus subtilis. CwlJ with a C-terminal His tag was functional and was extracted from spores by procedures that remove spore coat proteins. However, this CwlJ was not extracted from disrupted spores by dilute buffer, high salt concentrations, Triton X-100, Ca(2+)-dipicolinic acid, dithiothreitol, or peptidoglycan digestion, disappeared during spore germination, and was not present in cotE spores in which the spore coat is aberrant. These findings indicate the following: (i) the reason decoated and cotE spores germinate poorly with dipicolinic acid is the absence of CwlJ from these spores; and (ii) CwlJ is located in the spore coat, presumably tightly associated with one or more other coat proteins.
Figures


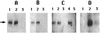
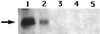
References
-
- Boland, F. M., A. Atrih, H. Chirakkal, S. J. Foster, and A. Moir. 2000. Complete spore-cortex hydrolysis during germination of Bacillus subtilis 168 requires SleB and YpeB. Microbiology 146:57-64. - PubMed
-
- Driks, A., and P. Setlow. 1999. Morphogenesis and properties of the bacterial spore, p. 191-218. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. American Society for Microbiology, Washington, D.C.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

