A quantitative morphometric analysis of rat spinal cord remyelination following transplantation of allogenic Schwann cells
- PMID: 11807836
- PMCID: PMC2605379
- DOI: 10.1002/cne.10117
A quantitative morphometric analysis of rat spinal cord remyelination following transplantation of allogenic Schwann cells
Abstract
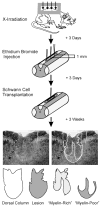
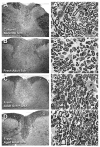
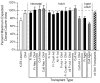
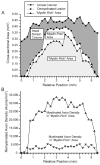
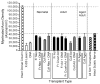
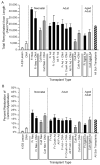
Quantitative morphometric techniques were used to assess the extent and pattern of remyelination produced by transplanting allogenic Schwann cells into demyelinated lesions in adult rat spinal cords. The effects of donor age, prior culturing of donor cells, prior lesioning of donor nerves, and host immunosuppression were evaluated by transplanting suspensions of 30,000 acutely dissociated or cultured Schwann cells from neonatal, young adult, or aged adult rat sciatic nerves into X-irradiation and ethidium bromide-induced demyelinated dorsal column lesions, with or without co-transplantation of neonatal optic nerve astrocytes. Three weeks after transplantation, spinal cords were processed for histological analysis. Under all Schwann cell transplant protocols, large areas containing many Schwann cell-like myelinated axon profiles could be readily observed throughout most of the lesion length. Within these "myelin-rich" regions, the vast majority of detectable axons showed a peripheral-like pattern of myelination. However, interaxonal spacing also increased, resulting in densities of myelinated axons that were more similar to peripheral nerve than intact dorsal columns. Freshly isolated Schwann cells remyelinated more axonal length than cultured Schwann cells, and cells from younger donors remyelinated slightly more axon length than cells from older donors, but all Schwann cell transplant protocols remyelinated tens of thousands of millimeters of axon length and remyelinated axons at similar densities. These results indicate that Schwann cells prepared under a variety of conditions are capable of eliciting remyelination, but that the density of remyelinated axons is much lower than the myelinated axon density in intact spinal cords.
Copyright 2002 Wiley-Liss, Inc.
Figures


Similar articles
-
Schwann cell remyelination is restricted to astrocyte-deficient areas after transplantation into demyelinated adult rat brain.J Neurosci Res. 2000 Jun 1;60(5):571-8. doi: 10.1002/(SICI)1097-4547(20000601)60:5<571::AID-JNR1>3.0.CO;2-Q. J Neurosci Res. 2000. PMID: 10820427
-
Transplanted neural stem/progenitor cells generate myelinating oligodendrocytes and Schwann cells in spinal cord demyelination and dysmyelination.Exp Neurol. 2008 Sep;213(1):176-90. doi: 10.1016/j.expneurol.2008.05.024. Epub 2008 Jun 10. Exp Neurol. 2008. PMID: 18586031
-
Schwann cell remyelination is not replaced by oligodendrocyte remyelination following ethidium bromide induced demyelination.Neuroreport. 2002 Jul 2;13(9):1205-8. doi: 10.1097/00001756-200207020-00027. Neuroreport. 2002. PMID: 12151770
-
Neural precursors as a cell source to repair the demyelinated spinal cord.J Neurotrauma. 2004 Apr;21(4):441-9. doi: 10.1089/089771504323004584. J Neurotrauma. 2004. PMID: 15115593 Review.
-
Promoting axonal myelination for improving neurological recovery in spinal cord injury.J Neurotrauma. 2009 Oct;26(10):1847-56. doi: 10.1089/neu.2008.0551. J Neurotrauma. 2009. PMID: 19785544 Review.
Cited by
-
Integration of engrafted Schwann cells into injured peripheral nerve: axonal association and nodal formation on regenerated axons.Neurosci Lett. 2005 Oct 21;387(2):85-9. doi: 10.1016/j.neulet.2005.06.073. Neurosci Lett. 2005. PMID: 16084645 Free PMC article.
-
Salidroside promotes peripheral nerve regeneration based on tissue engineering strategy using Schwann cells and PLGA: in vitro and in vivo.Sci Rep. 2017 Jan 5;7:39869. doi: 10.1038/srep39869. Sci Rep. 2017. PMID: 28054637 Free PMC article.
-
Soluble Neuregulin and Schwann Cell Myelination: a Therapeutic Potential for Improving Remyelination of Adult Axons.Mol Cell Pharmacol. 2010;2(4):161-167. doi: 10.4255/mcpharmacol.10.22. Mol Cell Pharmacol. 2010. PMID: 21274416 Free PMC article.
-
Multiple sclerosis: can Schwann cells wrap it up?Yale J Biol Med. 2002 Mar-Apr;75(2):113-6. Yale J Biol Med. 2002. PMID: 12230310 Free PMC article. Review. No abstract available.
-
Chronic TNFα Exposure Induces Robust Proliferation of Olfactory Ensheathing Cells, but not Schwann Cells.Neurochem Res. 2017 Sep;42(9):2595-2609. doi: 10.1007/s11064-017-2285-x. Epub 2017 May 11. Neurochem Res. 2017. PMID: 28497341
References
-
- Barnett SC, Alexander CL, Iwashita Y, Gilson JM, Crowther J, Clark L, Dunn LT, Papanastassiou V, Kennedy PG, Franklin RJ. Identification of a human olfactory ensheathing cell that can effect transplant-mediated remyelination of demyelinated CNS axons. Brain. 2000;123:1581–1588. - PubMed
-
- Baron-Van Evercooren A. Schwann call transplantation and myelin repair of the CNS. Multiple Sclerosis. 1997;3:157–161. - PubMed
-
- Baron-Van Evercooren A, Gansmuller A, Duhamel E, Pascal F, Gumpel M. Repair of a myelin lesion by Schwann cells transplanted into the adult mouse spinal cord. J Neuroimunol. 1992;40:235–242. - PubMed
-
- Baron van Evercooren A, Duhameal-Clerin E, Bounty JM, Hauw JJ, Gumpel M. Pathways of migration of transplanted Schwann cells in the demyelinated mouse spinal cord. J Neurosci Res. 1993;35:428–438. - PubMed
-
- Berthold CH. Morphology of normal peripheral axons. In: Waxman SG, editor. Physiology and pathobiology of axons. New York: Raven Press; 1978. pp. 3–64.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

