Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate
- PMID: 11808865
- PMCID: PMC2254176
- DOI: 10.1007/BF03226377
Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate
Abstract
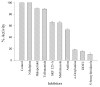
The aim of our study was to determine which microsomal cytochrome P450 isozyme(s) were responsible for the microsomal oxidation of indole to indoxyl, an important intermediate in the information of the uremic toxin indoxyl sulfate. Indole was incubated together with an NADPH-generating system and rat liver microsomes. Formation of indigo, an auto-oxidation product of indoxyl, was used to determine the indole-3-hydroxylation activity. Apparent Km and Vmax values of 0.85 mM and 1152 pmol min(-1) mg(-1) were calculated for the formation of indoxyl from indole using rat liver microsomes. The effects of various potential inducers and inhibitors on the metabolism of indole to indoxyl by rat liver microsomes were studied to elucidate the enzymes responsible for metabolism. Studies with general and isozyme-specific P450 inhibitors demostrated that P450 enzymes and not FMO are responsible for the formation of indoxyl. In the induction studies, rate of indoxyl formation in the microsomes from untreated vs induced rats correlated nearly exactly with the CYP2E1 activity (4-nitrophenol 2-hydroxylation). These results suggests that CYP2E1 is the major isoform for the microsomal oxidation of indole to indoxyl.
Figures



References
-
- Bryan GT. The role of urinary tryptophan metabolites in the etiology of bladder cancer. Am. J. Clin. Nutr. 1971;24:841–847. - PubMed
-
- Dunning WF, Curtis MR. The role of indole in incidence of 2-acetylaminofluorene-induced bladder cancer in rats. Proc. Soc. Exp. Biol. Med. 1958;99:91–95. - PubMed
-
- Oyasu R, Kitajima T, Hopp ML, Sumie H. Enhancement of urinary bladder tumorigenesis in hamsters by coadministration of 2-acetylaminofluorene and indole. Cancer Res. 1972;32:2027–2033. - PubMed
-
- Sims J, Renwick AG. The effects of saccharin on the metabolism of dietary tryptophan to indole, a known cocarcinogen for the urinary bladder of the rat. Toxicol. Appl. Pharmacol. 1983;67:132–151. - PubMed
-
- Lawrie CA, Renwick AG, Sims J. The urinary excretion of bacterial amino-acid metabolites by rats fed saccharin in the diet. Food Chem. Toxicol. 1985;23:445–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
