Unique motif for nucleolar retention and nuclear export regulated by phosphorylation
- PMID: 11809804
- PMCID: PMC134639
- DOI: 10.1128/MCB.22.4.1126-1139.2002
Unique motif for nucleolar retention and nuclear export regulated by phosphorylation
Abstract
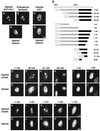
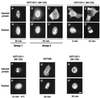
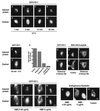
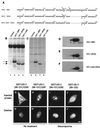
By microinjecting purified glutathione S-transferase linked to all or parts of herpes simplex virus type 1 US11 protein into either the nucleus or the cytoplasm, we have demonstrated that this nucleolar protein exhibits a new type of localization signal controlling both retention in nucleoli and export to the cytoplasm. Saturated mutagenesis combined with computer modeling allowed us to draw the fine-structure map of this domain, revealing a new proline-rich motif harboring both activities, which are temperature dependent and regulated by phosphorylation. Finally, crossing the nuclear pore complex from the cytoplasm to the nucleus is an energy-dependent process for US11 protein, while getting to nucleoli through the nucleoplasm is energy independent.
Figures


References
-
- Besse, S., J.-J. Diaz, E. Pichard, K. Kindbeiter, J.-J. Madjar, and F. Puvion-Dutilleul. 1996. In situ hybridization and immunoelectron microscope analyses of the Us11 gene of herpes simplex virus type 1 for transient expression. Chromosoma 104:434-444. - PubMed
-
- Bogerd, H. P., R. E. Benson, R. Truant, A. Herold, M. Phingbodhipakkiya, and B. R. Cullen. 1999. Definition of a consensus transportin-specific nucleocytoplasmic transport signal. J. Biol. Chem. 274:9771-9777. - PubMed
-
- Boulikas, T. 1993. Nuclear localization signals (NLS). Crit. Rev. Eukaryot. Gene Expr. 3:193-227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
