Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein traffic
- PMID: 11809827
- PMCID: PMC65077
- DOI: 10.1091/mbc.01-08-0420
Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein traffic
Abstract
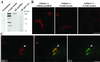
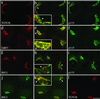
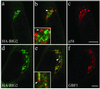
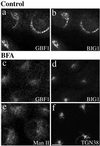
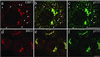
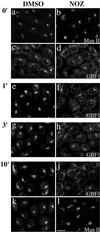
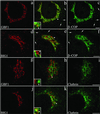
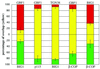
Activation of several ADP-ribosylation factors (ARFs) by guanine nucleotide exchange factors (GEFs) regulates recruitment of coat proteins (COPs) on the Golgi complex and is generally assumed to be the target of brefeldin A (BFA). The large ARF-GEFs Golgi-specific BFA resistance factor 1 (GBF1) and BFA-inhibited GEFs (BIGs) localize to this organelle but catalyze exchange preferentially on class II and class I ARFs, respectively. We now demonstrate using quantitative confocal microscopy that these GEFs show a very limited overlap with each other (15 and 23%). In contrast, GBF1 colocalizes with the cis-marker p115 (86%), whereas BIGs overlap extensively with TGN38 (83%). Consistent with these distributions, GBF1, but not BIG1, partially relocalized to peripheral sites after incubation at 15 degrees C. The new GBF1 structures represent peripheral vesicular tubular clusters (VTCs) because 88% of structures analyzed stained for both GBF1 and p115. Furthermore, as expected of VTCs, they rapidly reclustered to the Golgi complex in a microtubule-dependent manner upon warm-up. These observations suggest that GBF1 and BIGs activate distinct subclasses of ARFs in specific locations to regulate different types of reactions. In agreement with this possibility, COPI overlapped to a greater extent with GBF1 (64%) than BIG1 (31%), whereas clathrin showed limited overlap with BIG1, and virtually none with GBF1.
Figures

References
-
- Antonny B, Schekman R. ER export: public transportation by the COPII coach. Curr Opin Cell Biol. 2001;13:438–443. - PubMed
-
- Brown HA, Gutowsk S, Moomaw CR, Slaughter C, Sternweis PC. ADP- ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

