Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles
- PMID: 11809831
- PMCID: PMC65080
- DOI: 10.1091/mbc.01-07-0380
Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles
Abstract
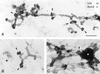
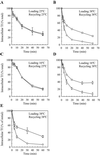
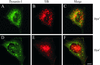
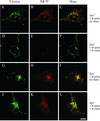
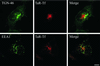
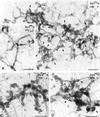
Previously we described clathrin-coated buds on tubular early endosomes that are distinct from those at the plasma membrane and the trans-Golgi network. Here we show that these clathrin-coated buds, like plasma membrane clathrin-coated pits, contain endogenous dynamin-2. To study the itinerary that is served by endosome-derived clathrin-coated vesicles, we used cells that overexpressed a temperature-sensitive mutant of dynamin-1 (dynamin-1(G273D)) or, as a control, dynamin-1 wild type. In dynamin-1(G273D)-expressing cells, 29-36% of endocytosed transferrin failed to recycle at the nonpermissive temperature and remained associated with tubular recycling endosomes. Sorting of endocytosed transferrin from fluid-phase endocytosed markers in early endosome antigen 1-labeled sorting endosomes was not inhibited. Dynamin-1(G273D) associated with accumulated clathrin-coated buds on extended tubular recycling endosomes. Brefeldin A interfered with the assembly of clathrin coats on endosomes and reduced the extent of transferrin recycling in control cells but did not further affect recycling by dynamin-1(G273D)-expressing cells. Together, these data indicate that the pathway from recycling endosomes to the plasma membrane is mediated, at least in part, by endosome-derived clathrin-coated vesicles in a dynamin-dependent manner.
Figures




References
-
- Baba T, Ueda H, Terada N, Fujii Y, Ohno S. Immunocytochemical study of endocytotic structures accumulated in HeLa cells transformed with a temperature-sensitive mutant of dynamin. J Histochem Cytochem. 1999;47:637–648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

