Fission yeast F-box protein Pof3 is required for genome integrity and telomere function
- PMID: 11809834
- PMCID: PMC65083
- DOI: 10.1091/mbc.01-07-0333
Fission yeast F-box protein Pof3 is required for genome integrity and telomere function
Abstract
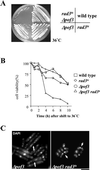
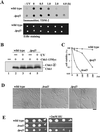
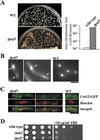
The Skp1-Cullin-1/Cdc53-F-box protein (SCF) ubiquitin ligase plays an important role in various biological processes. In this enzyme complex, a variety of F-box proteins act as receptors that recruit substrates. We have identified a fission yeast gene encoding a novel F-box protein Pof3, which contains, in addition to the F-box, a tetratricopeptide repeat motif in its N terminus and a leucine-rich-repeat motif in the C terminus, two ubiquitous protein-protein interaction domains. Pof3 forms a complex with Skp1 and Pcu1 (fission yeast cullin-1), suggesting that Pof3 functions as an adaptor for specific substrates. In the absence of Pof3, cells exhibit a number of phenotypes reminiscent of genome integrity defects. These include G2 cell cycle delay, hypersensitivity to UV, appearance of lagging chromosomes, and a high rate of chromosome loss. pof3 deletion strains are viable because the DNA damage checkpoint is continuously activated in the mutant, and this leads to G2 cell cycle delay, thereby preventing the mutant from committing lethal mitosis. Pof3 localizes to the nucleus during the cell cycle. Molecular analysis reveals that in this mutant the telomere is substantially shortened and furthermore transcriptional silencing at the telomere is alleviated. The results highlight a role of the SCF(Pof3) ubiquitin ligase in genome integrity via maintaining chromatin structures.
Figures





Similar articles
-
Molecular interactions of fission yeast Skp1 and its role in the DNA damage checkpoint.Genes Cells. 2004 May;9(5):367-82. doi: 10.1111/j.1356-9597.2004.00730.x. Genes Cells. 2004. PMID: 15147268
-
Two F-box/WD-repeat proteins Pop1 and Pop2 form hetero- and homo-complexes together with cullin-1 in the fission yeast SCF (Skp1-Cullin-1-F-box) ubiquitin ligase.Genes Cells. 1998 Nov;3(11):721-35. doi: 10.1046/j.1365-2443.1998.00225.x. Genes Cells. 1998. PMID: 9990507
-
Hsk1- and SCF(Pof3)-dependent proteolysis of S. pombe Ams2 ensures histone homeostasis and centromere function.Dev Cell. 2010 Mar 16;18(3):385-96. doi: 10.1016/j.devcel.2009.12.024. Dev Cell. 2010. PMID: 20230746 Free PMC article.
-
The F-box: a new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction.Prog Biophys Mol Biol. 1999;72(3):299-328. doi: 10.1016/s0079-6107(99)00010-3. Prog Biophys Mol Biol. 1999. PMID: 10581972 Review.
-
The F-box protein family.Genome Biol. 2000;1(5):REVIEWS3002. doi: 10.1186/gb-2000-1-5-reviews3002. Epub 2000 Nov 10. Genome Biol. 2000. PMID: 11178263 Free PMC article. Review.
Cited by
-
Genome-wide screen of genes required for caffeine tolerance in fission yeast.PLoS One. 2009 Aug 12;4(8):e6619. doi: 10.1371/journal.pone.0006619. PLoS One. 2009. PMID: 19672306 Free PMC article.
-
New insights into donor directionality of mating-type switching in Schizosaccharomyces pombe.PLoS Genet. 2018 May 31;14(5):e1007424. doi: 10.1371/journal.pgen.1007424. eCollection 2018 May. PLoS Genet. 2018. PMID: 29852001 Free PMC article.
-
CAND1 controls in vivo dynamics of the cullin 1-RING ubiquitin ligase repertoire.Nat Commun. 2013;4:1642. doi: 10.1038/ncomms2636. Nat Commun. 2013. PMID: 23535663 Free PMC article.
-
Proteasome-dependent degradation of replisome components regulates faithful DNA replication.Cell Cycle. 2013 Aug 15;12(16):2564-9. doi: 10.4161/cc.25692. Epub 2013 Jul 18. Cell Cycle. 2013. PMID: 23907116 Free PMC article. Review.
-
F-box-directed CRL complex assembly and regulation by the CSN and CAND1.Mol Cell. 2009 Sep 11;35(5):586-97. doi: 10.1016/j.molcel.2009.07.024. Mol Cell. 2009. PMID: 19748355 Free PMC article.
References
-
- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 1995;9:218–233. - PubMed
-
- Bähler J, Wu J, Longtine MS, Shah NG, McKenzie A, III, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. - PubMed
-
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. - PubMed
-
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

