Absence of direct delivery for single transmembrane apical proteins or their "Secretory" forms in polarized hepatic cells
- PMID: 11809835
- PMCID: PMC65084
- DOI: 10.1091/mbc.01-07-0376
Absence of direct delivery for single transmembrane apical proteins or their "Secretory" forms in polarized hepatic cells
Abstract
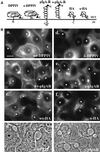
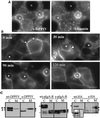
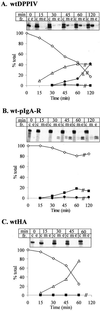
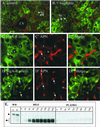
The absence of a direct route to the apical plasma membrane (PM) for single transmembrane domain (TMD) proteins in polarized hepatic cells has been inferred but never directly demonstrated. The genes encoding three pairs of apical PM proteins, whose extracellular domains are targeted exclusively to the apical milieu in Madin-Darby canine kidney cells, were packaged into recombinant adenovirus and delivered to WIF-B cells in vitro and liver hepatocytes in vivo. By immunofluorescence and pulse-chase metabolic labeling, we found that the soluble constructs were overwhelmingly secreted into the basolateral milieu, which in vivo is the blood and in vitro is the culture medium. The full-length proteins were first delivered to the basolateral surface but then concentrated in the apical PM. Our results imply that hepatic cells lack trans-Golgi network (TGN)-based machinery for directly sorting single transmembrane domain apical proteins and raise interesting questions about current models of PM protein sorting in polarized and nonpolarized cells.
Figures

References
-
- Aroeti B, Okhrimenko H, Reich V, Orzech E. Polarized trafficking of plasma membrane proteins: emerging roles for coats, SNAREs, GTPases and their link to the cytoskeleton. Biochim Biophys Acta. 1998;1376:57–90. - PubMed
-
- Barr VA, Hubbard AL. Newly synthesized hepatocyte plasma membrane proteins are transported in transcytotic vesicles in the bile duct-ligated rat. Gastroenterology. 1993;105:554–571. - PubMed
-
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. - PubMed
-
- Casanova JE, Hubbard AL, Mishumi Y, Ikehara Y, Mostov KE. Direct apical sorting of rat liver dipeptidylpeptidase IV expressed in MDCK cells. J Biol Chem. 1991;266:24428–24432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

