Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking
- PMID: 11809836
- PMCID: PMC65085
- DOI: 10.1091/mbc.01-06-0317
Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking
Abstract
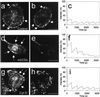
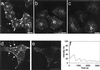
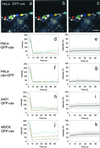
To investigate whether caveolae are involved in constitutive endocytic trafficking, we expressed N- and C- terminally green fluorescent protein (GFP)-tagged caveolin- 1 fusion proteins in HeLa, A431, and Madin-Darby canine kidney cells. The fusion proteins were shown by immunogold labeling to be sorted correctly to caveolae. By using confocal microscopy and photobleaching techniques, it was found that although intracellular structures labeled with GFP-tagged caveolin were dynamic, GFP-labeled caveolae were very immobile. However, after incubation with methyl- beta-cyclodextrin, distinct caveolae disappeared and the mobility of GFP-tagged caveolin in the plasma membrane increased. Treatment of cells with cytochalasin D caused lateral movement and aggregation of GFP-labeled caveolae. Therefore, both cholesterol and an intact actin cytoskeleton are required for the integrity of GFP-labeled caveolae. Moreover, stimulation with okadaic acid caused increased mobility and internalization of the labeled caveolae. Although the calculated mobile fraction (for t = infinity) of intracellular, GFP-tagged caveolin- associated structures was 70-90%, GFP-labeled caveolae in unstimulated cells had a mobile fraction of <20%, a value comparable to that previously reported for E-cadherin in junctional complexes. We therefore conclude that caveolae are not involved in constitutive endocytosis but represent a highly stable plasma membrane compartment anchored by the actin cytoskeleton.
Figures






References
-
- Anderson RGW. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. - PubMed
-
- Bucci M, Gratton J-P, Rudic RD, Acevedo L, Roviezzo F, Crino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis, and reduces inflammation. Nat Med. 2000b;6:1362–1367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

