Biogenesis of N-cadherin-dependent cell-cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism
- PMID: 11809840
- PMCID: PMC65089
- DOI: 10.1091/mbc.01-07-0337
Biogenesis of N-cadherin-dependent cell-cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism
Abstract
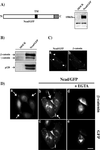
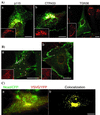
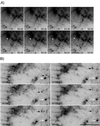
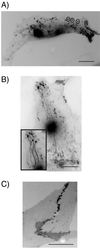
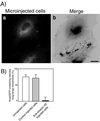
Cadherin-mediated cell-cell adhesion is a dynamic process that is regulated during embryonic development, cell migration, and differentiation. Different cadherins are expressed in specific tissues consistent with their roles in cell type recognition. In this study, we examine the formation of N-cadherin-dependent cell-cell contacts in fibroblasts and myoblasts. In contrast to E-cadherin, both endogenous and ectopically expressed N-cadherin shuttles between an intracellular and a plasma membrane pool. Initial formation of N-cadherin-dependent cell-cell contacts results from the recruitment of the intracellular pool of N-cadherin to the plasma membrane. N-cadherin also localizes to the Golgi apparatus and both secretory and endocytotic vesicles. We demonstrate that the intracellular pool of N-cadherin is tightly associated with the microtubule (MT) network and that junction formation requires MTs. In addition, localization of N-cadherin to the cortex is dependent on an intact F-actin cytoskeleton. We show that N-cadherin transport requires the MT network as well as the activity of the MT-associated motor kinesin. In conclusion, we propose that N-cadherin distribution is a regulated process promoted by cell-cell contact formation, which controls the biogenesis and turnover of the junctions through the MT network.
Figures




References
-
- Akitaya T, Bronner-Fraser M. Expression of cell adhesion molecules during initiation and cessation of neural crest cell migration. Dev Dyn. 1992;194:12–20. - PubMed
-
- Bronner-Fraser M. Mechanisms of neural crest cell migration. Bioessays. 1993;15:221–230. - PubMed
-
- Charrasse S, Schroeder M, Gauthier-Rouviere C, Ango F, Cassimeris L, Gard DL, Larroque C. The TOGp protein is a new human microtubule-associated protein homologous to the XenopusXMAP215. J Cell Sci. 1998;111:1371–1383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

