Suppression of genetic defects within the RAD6 pathway by srs2 is specific for error-free post-replication repair but not for damage-induced mutagenesis
- PMID: 11809886
- PMCID: PMC100297
- DOI: 10.1093/nar/30.3.732
Suppression of genetic defects within the RAD6 pathway by srs2 is specific for error-free post-replication repair but not for damage-induced mutagenesis
Abstract
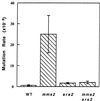
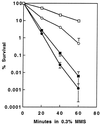
srs2 was isolated during a screen for mutants that could suppress the UV-sensitive phenotype of rad6 and rad18 cells. Genetic analyses led to a proposal that Srs2 acts to prevent the channeling of DNA replication-blocking lesions into homologous recombination. The phenotypes associated with srs2 indicate that the Srs2 protein acts to process lesions through RAD6-mediated post-replication repair (PRR) rather than recombination repair. The RAD6 pathway has been divided into three rather independent subpathways: two error-free (represented by RAD5 and POL30) and one error-prone (represented by REV3). In order to determine on which subpathways Srs2 acts, we performed comprehensive epistasis analyses; the experimental results indicate that the srs2 mutation completely suppresses both error-free PRR branches. Combined with UV-induced mutagenesis assays, we conclude that the Polzeta-mediated error-prone pathway is functional in the absence of Srs2; hence, Srs2 is not required for mutagenesis. Furthermore, we demonstrate that the helicase activity of Srs2 is probably required for the phenotypic suppression of error-free PRR defects. Taken together, our observations link error-free PRR to homologous recombination through the helicase activity of Srs2.
Figures




References
-
- di Caprio L. and Cox,B.S. (1981) DNA synthesis in UV-irradiated yeast. Mutat. Res., 82, 69–85. - PubMed
-
- Prakash L. (1981) Characterization of postreplication repair in Saccharomyces cerevisiae and effects of rad6, rad18, rev3 and rad52 mutations. Mol. Gen. Genet., 184, 471–478. - PubMed
-
- Nelson J.R., Lawrence,C.W. and Hinkle,D.C. (1996) Thymine-thymine dimer bypass by yeast DNA polymerase ζ. Science, 272, 1646–1649. - PubMed
-
- Broomfield S., Hryciw,T. and Xiao,W. (2001) DNA postreplication repair and mutagenesis in Saccharomyces cerevisiae. Mutat. Res., 486, 167–184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

