Padlock oligonucleotides as a tool for labeling superhelical DNA
- PMID: 11809900
- PMCID: PMC100311
- DOI: 10.1093/nar/30.3.e12
Padlock oligonucleotides as a tool for labeling superhelical DNA
Abstract
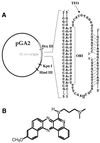
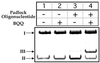
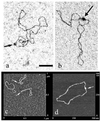
Labeling of a covalently closed circular double-stranded DNA was achieved using a so-called 'padlock oligonucleotide'. The oligonucleotide was targeted to a sequence which is present in the replication origin of phage f1 and thus in numerous commonly used plasmids. After winding around the double-stranded target DNA sequence by ligand-induced triple helix formation, a biotinylated oligonucleotide was circularized using T4 DNA ligase and in this way became catenated to the plasmid. A gel shift assay was developed to measure the extent of plasmid modification by the padlock oligonucleotide. A similar assay showed that a modified supercoiled plasmid was capable of binding one streptavidin molecule thanks to the biotinylated oligonucleotide and that this binding was quantitative. The catenated complex was visualized by electron and atomic force microscopies using streptavidin conjugates or single strand-binding proteins as protein tags for the padlock oligonucleotide. This method provides a versatile tool for plasmid functionalization which offers new perspectives in the physical study of supercoiled DNA and in the development of improved vectors for gene therapy.
Figures

Similar articles
-
Padlock oligonucleotides for duplex DNA based on sequence-specific triple helix formation.Proc Natl Acad Sci U S A. 1999 Sep 14;96(19):10603-7. doi: 10.1073/pnas.96.19.10603. Proc Natl Acad Sci U S A. 1999. PMID: 10485872 Free PMC article.
-
Sequence-specific labeling of superhelical DNA by triple helix formation and psoralen crosslinking.Nucleic Acids Res. 1996 May 1;24(9):1702-9. doi: 10.1093/nar/24.9.1702. Nucleic Acids Res. 1996. PMID: 8649989 Free PMC article.
-
Unpaired structures in SCA10 (ATTCT)n.(AGAAT)n repeats.J Mol Biol. 2003 Feb 28;326(4):1095-111. doi: 10.1016/s0022-2836(03)00037-8. J Mol Biol. 2003. PMID: 12589756
-
Supramolecular DNA-streptavidin nanocircles with a covalently attached oligonucleotide moiety.J Biomol Struct Dyn. 2002 Oct;20(2):223-30. doi: 10.1080/07391102.2002.10506838. J Biomol Struct Dyn. 2002. PMID: 12354074
-
Topological Behavior of Plasmid DNA.Microbiol Spectr. 2015 Apr;3(2):10.1128/microbiolspec.PLAS-0036-2014. doi: 10.1128/microbiolspec.PLAS-0036-2014. Microbiol Spectr. 2015. PMID: 26104708 Free PMC article. Review.
Cited by
-
Applications of triplex DNA nanostructures in sensor development.Anal Bioanal Chem. 2022 Jul;414(18):5217-5237. doi: 10.1007/s00216-022-04058-8. Epub 2022 Apr 25. Anal Bioanal Chem. 2022. PMID: 35469098 Review.
-
Sequence-specific fluorescent labeling of double-stranded DNA observed at the single molecule level.Nucleic Acids Res. 2003 Oct 15;31(20):e125. doi: 10.1093/nar/gng125. Nucleic Acids Res. 2003. PMID: 14530458 Free PMC article.
-
The triple helix: 50 years later, the outcome.Nucleic Acids Res. 2008 Sep;36(16):5123-38. doi: 10.1093/nar/gkn493. Epub 2008 Aug 1. Nucleic Acids Res. 2008. PMID: 18676453 Free PMC article. Review.
-
Labeling of unique sequences in double-stranded DNA at sites of vicinal nicks generated by nicking endonucleases.Nucleic Acids Res. 2008 Apr;36(7):e40. doi: 10.1093/nar/gkn107. Epub 2008 Mar 15. Nucleic Acids Res. 2008. PMID: 18344522 Free PMC article.
-
A capture approach for supercoiled plasmid DNA using a triplex-forming oligonucleotide.Nucleic Acids Res. 2013 May 1;41(10):e111. doi: 10.1093/nar/gkt239. Epub 2013 Apr 9. Nucleic Acids Res. 2013. PMID: 23571753 Free PMC article.
References
-
- Ciolina C., Byk,G., Blanche,F., Thuillier,V., Scherman,D. and Wils,P. (1999) Coupling of nuclear localization signals to plasmid DNA and specific interaction of the conjugates with importin alpha. Bioconjugate Chem., 10, 49–55. - PubMed
-
- Neves C., Byk,G., Escriou,V., Bussone,F., Scherman,D. and Wils,P. (2000) Novel method for covalent fluorescent labeling of plasmid DNA that maintains structural integrity of the plasmid. Bioconjugate Chem., 11, 51–55. - PubMed
-
- Leahy P., Carmichael,G.G. and Rossomando,E.F. (1996) Novel biotinylated plasmid expression vectors retain biological function and can bind streptavidin. Bioconjugate Chem., 7, 545–551. - PubMed
-
- Sebestyen M.G., Ludtke,J.J., Bassik,M.C., Zhang,G., Budker,V., Lukhtanov,E.A., Hagstrom,J.E. and Wolff,J.A. (1998) DNA vector chemistry: the covalent attachment of signal peptides to plasmid DNA. Nat. Biotechnol., 16, 80–85. - PubMed

