Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye
- PMID: 11815344
- PMCID: PMC1771021
- DOI: 10.1136/bjo.86.2.181
Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye
Abstract
Background/aims: Several studies have reported that sodium hyaluronate is able to improve both symptoms and signs in patients with dry eye but none have demonstrated an improvement of conjunctival epithelial cell abnormalities of the ocular surface. The aim of this study was to explore the effect of sodium hyaluronate-containing eye drops on the ocular surface of patients with dry eye during long term treatment.
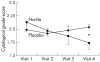
Methods: A randomised double blind study was undertaken in 86 patients with medium to severe dry eye (that is, rose bengal and/or fluorescein test score of at least 3, tear film break up time <10 seconds, or Schirmer's test <5.5 mm). Patients were treated with either preservative-free sodium hyaluronate or saline for 3 months at a dose of one drop 4-8 times a day. Bulbar impression cytology, slit lamp examinations, and subjective symptoms were evaluated after 1, 2, and 3 months. Impression cytology was considered the primary efficacy parameter of the study.
Results: The efficacy analysis was performed on a total of 44 patients who were able to fully adhere to the protocol. After 3 months of treatment sodium hyaluronate improved impression cytology score (p = 0.024 v baseline). At the same time also the difference with respect to placebo was statistically significant (p = 0.036). Study medication was well tolerated and no treatment related adverse events occurred during the study.
Conclusions: Sodium hyaluronate may effectively improve ocular surface damage associated with dry eye syndrome.
Figures
References
-
- Nelson JD, Havener VR, Cameron JD. Cellulose acetate impressions of the ocular surface. Arch Ophthalmol 1983;101:1868–72. - PubMed
-
- Lemp MA. Artificial tear solutions. Int Ophthalmol Clin 1973;13:221–9. - PubMed
-
- De Luise VP, Peterson SW. The use of topical Healon tears in the management of refractory dry-eye syndrome. Ann Ophthalmol 1984;16:823–4. - PubMed
-
- Stuart JC, Linn JG. Dilute sodium hyaluronate (Healon) in the treatment of ocular surface disorders. Ann Ophthalmol 1985;17:190–2 - PubMed
-
- Polack FM, McNiece. The treatment of dry-eyes with Na-hyaluronate (Healon). Cornea 1982;1:133–6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
