Andrographolide prevents oxygen radical production by human neutrophils: possible mechanism(s) involved in its anti-inflammatory effect
- PMID: 11815375
- PMCID: PMC1573154
- DOI: 10.1038/sj.bjp.0704493
Andrographolide prevents oxygen radical production by human neutrophils: possible mechanism(s) involved in its anti-inflammatory effect
Abstract
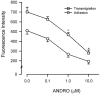
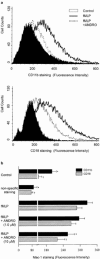
We have reported that andrographolide (ANDRO), an active component of Andrographis paniculata, inhibits inflammatory responses by rat neutrophils. To further elucidate the possible mechanism(s) underlying the ANDRO's effect, N-formyl-methionyl-leucyl-phenylalanine (fMLP)-induced adhesion and transmigration of isolated peripheral human neutrophils were studied. Pretreatment with ANDRO (0.1 - 10 microM) concentration-dependently prevented fMLP-induced neutrophil adhesion and transmigration. We further examined the up-expression of surface Mac-1 (CD11b/CD18), an essential integrin mediated in neutrophil adhesion and transmigration. ANDRO pretreatment significantly decreased fMLP-induced up-expression of both CD11b and CD18. Accumulation of reactive oxygen species (ROS) as well as quick intracellular calcium ([Ca(++)](i)) mobilization induced by fMLP displays two important signalling pathways in regulating the up-expression of Mac-1 by neutrophils. That ANDRO pretreatment diminished fMLP-induced production of H(2)O(2) and O(2)*(-), but failed to block that of [Ca(++)](i) mobilization suggested that the ROS but not [Ca(++)](i) signalling could be modulated by ANDRO. To clarify whether ROS production impeded by ANDRO could be an antagonism of fMLP binding, phorbol-12-myristate-13-acetate (PMA), a direct protein kinase C (PKC) activator, was introduced to activate ROS production. PMA triggered remarkable ROS production and adhesion, and were partially reversed by ANDRO. This indicated that a PKC-dependent mechanism might be interfered by ANDRO. We conclude that the prevention of ROS production through, at least in part, modulation of PKC-dependent pathway could confer ANDRO the ability to down-regulate Mac-1 up-expression that is essential for neutrophil adhesion and transmigration.
Figures



Similar articles
-
Prevention of macrophage adhesion molecule-1 (Mac-1)-dependent neutrophil firm adhesion by taxifolin through impairment of protein kinase-dependent NADPH oxidase activation and antagonism of G protein-mediated calcium influx.Biochem Pharmacol. 2004 Jun 15;67(12):2251-62. doi: 10.1016/j.bcp.2004.02.020. Biochem Pharmacol. 2004. PMID: 15163556
-
Impediment to calcium influx and reactive oxygen production accounts for the inhibition of neutrophil Mac-1 Up-regulation and adhesion by tetrandrine.Mol Pharmacol. 1999 Jan;55(1):186-93. doi: 10.1124/mol.55.1.186. Mol Pharmacol. 1999. PMID: 9882713
-
Suppression of rat neutrophil reactive oxygen species production and adhesion by the diterpenoid lactone andrographolide.Planta Med. 2000 May;66(4):314-7. doi: 10.1055/s-2000-8537. Planta Med. 2000. PMID: 10865445
-
Anti-inflammatory effects of the partially purified extract of radix Stephaniae tetrandrae: comparative studies of its active principles tetrandrine and fangchinoline on human polymorphonuclear leukocyte functions.Mol Pharmacol. 2001 Nov;60(5):1083-90. Mol Pharmacol. 2001. PMID: 11641437
-
Andrographolide: A promising therapeutic agent against organ fibrosis.Eur J Med Chem. 2024 Dec 15;280:116992. doi: 10.1016/j.ejmech.2024.116992. Epub 2024 Oct 20. Eur J Med Chem. 2024. PMID: 39454221 Review.
Cited by
-
Neurobiological Promises of the Bitter Diterpene Lactone Andrographolide.Oxid Med Cell Longev. 2022 Feb 1;2022:3079577. doi: 10.1155/2022/3079577. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35154564 Free PMC article. Review.
-
Andrographis paniculata Formulations: Impact on Diterpene Lactone Oral Bioavailability.Eur J Drug Metab Pharmacokinet. 2022 Jan;47(1):19-30. doi: 10.1007/s13318-021-00736-7. Epub 2021 Nov 23. Eur J Drug Metab Pharmacokinet. 2022. PMID: 34816382 Free PMC article. Review.
-
Antitumor Potential of Immunomodulatory Natural Products.Mar Drugs. 2022 Jun 8;20(6):386. doi: 10.3390/md20060386. Mar Drugs. 2022. PMID: 35736189 Free PMC article. Review.
-
Therapeutic Potential of Andrographolide Isolated from the Leaves of Andrographis paniculata Nees for Treating Lung Adenocarcinomas.Evid Based Complement Alternat Med. 2013;2013:305898. doi: 10.1155/2013/305898. Epub 2013 Aug 20. Evid Based Complement Alternat Med. 2013. PMID: 23997793 Free PMC article.
-
Hepatoprotective Effects of Chinese Medicinal Herbs: A Focus on Anti-Inflammatory and Anti-Oxidative Activities.Int J Mol Sci. 2016 Mar 29;17(4):465. doi: 10.3390/ijms17040465. Int J Mol Sci. 2016. PMID: 27043533 Free PMC article. Review.
References
-
- ALBELDA S.M., SMITH C.W., WARD P.A. Adhesion molecules and inflammatory injury. FASEB J. 1994;8:504–512. - PubMed
-
- AMROYAN E., GABRIELIAN E., PANOSSIAN A., WIKMAN G., WAGNER H. Inhibitory effect of andrographolide from Andrographis paniculata on PAF-induced platelet aggregation. Phytomedicine. 1999;6:27–31. - PubMed
-
- ARFORS K.E., LUNDBERG C., LINDBOM L., LUNDBERG K., BEATTY P.G., HARLAN J.M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987;69:338–340. - PubMed
-
- ATTA U.R., HARVEY K., SIDDIQUI R.A. Interleukin-8: An autocrine inflammatory mediator. Curr. Pharmaceutical. Design. 1999;5:241–253. - PubMed
-
- BARRITT G.J., LEE A.M. Effects of electrical stimulation and an intracellular calcium chelator on calcium movement in suspensions of isolated myocardial muscle cells. Cardiovasc. Res. 1985;19:370–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

