Conductance and permeability of the residual state of connexin43 gap junction channels
- PMID: 11815667
- PMCID: PMC2233803
- DOI: 10.1085/jgp.119.2.171
Conductance and permeability of the residual state of connexin43 gap junction channels
Abstract
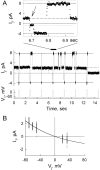
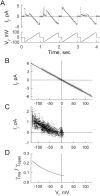
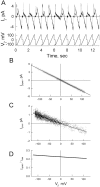
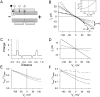
We used cell lines expressing wild-type connexin43 and connexin43 fused with the enhanced green fluorescent protein (Cx43-EGFP) to examine conductance and perm-selectivity of the residual state of Cx43 homotypic and Cx43/Cx43-EGFP heterotypic gap junction channels. Each hemichannel in Cx43 cell-cell channel possesses two gates: a fast gate that closes channels to the residual state and a slow gate that fully closes channels; the transjunctional voltage (V(j)) closes the fast gate in the hemichannel that is on the relatively negative side. Here, we demonstrate macroscopically and at the single-channel level that the I-V relationship of the residual state rectifies, exhibiting higher conductance at higher V(j)s that are negative on the side of gated hemichannel. The degree of rectification increases when Cl(-) is replaced by Asp(-) and decreases when K(+) is replaced by TEA(+). These data are consistent with an increased anionic selectivity of the residual state. The V(j)-gated channel is not permeable to monovalent positively and negatively charged dyes, which are readily permeable through the fully open channel. These data indicate that a narrowing of the channel pore accompanies gating to the residual state. We suggest that the fast gate operates through a conformational change that introduces positive charge at the cytoplasmic vestibule of the gated hemichannel, thereby producing current rectification, increased anionic selectivity, and a narrowing of channel pore that is largely responsible for reducing channel conductance and restricting dye transfer. Consequently, the fast V(j)-sensitive gating mechanism can serve as a selectivity filter, which allows electrical coupling but limits metabolic communication.
Figures
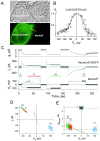
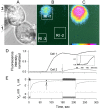
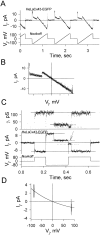
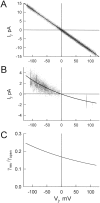


Comment in
-
Voltage-sensing and substate rectification: moving parts of connexin channels.J Gen Physiol. 2002 Feb;119(2):165-9. doi: 10.1085/jgp.119.2.165. J Gen Physiol. 2002. PMID: 11815666 Free PMC article. Review. No abstract available.
References
-
- Banach, K., and R. Weingart. 2000. Voltage gating of Cx43 gap junction channels involves fast and slow current transitions. Pflügers Arch. 439:248–250. - PubMed
-
- Bennett, M.V., T.A. Bargiello, L. Barrio, D.C. Spray, E.L. Hertzberg, and J.C. Sáez. 1991. Gap junctions: new tools, new answers, new questions. Neuron. 6:305–320. - PubMed
-
- Bukauskas, F. 2001. Inducing de novo formation of gap junction channels. Methods in Molecular Biology. Connexin: Methods and Protocols. Vol. 154. R. Bruzzone and C. Giaume, editors. Humana Press, Totowa, New Jersey. 379–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

