Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices
- PMID: 11815668
- PMCID: PMC2233801
- DOI: 10.1085/jgp.119.2.187
Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices
Abstract
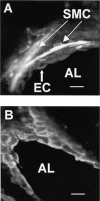
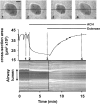
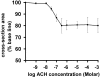
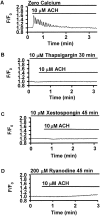
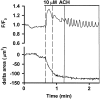
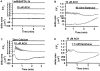
The Ca(2+) signaling and contractility of airway smooth muscle cells (SMCs) were investigated with confocal microscopy in murine lung slices (approximately 75-microm thick) that maintained the in situ organization of the airways and the contractility of the SMCs for at least 5 d. 10--500 nM acetylcholine (ACH) induced a contraction of the airway lumen and a transient increase in [Ca(2+)](i) in individual SMCs that subsequently declined to initiate multiple intracellular Ca(2+) oscillations. These Ca(2+) oscillations spread as Ca(2+) waves through the SMCs at approximately 48 microm/s. The magnitude of the airway contraction, the initial Ca(2+) transient, and the frequency of the subsequent Ca(2+) oscillations were all concentration-dependent. In a Ca(2+)-free solution, ACH induced a similar Ca(2+) response, except that the Ca(2+) oscillations ceased after 1--1.5 min. Incubation with thapsigargin, xestospongin, or ryanodine inhibited the ACH-induced Ca(2+) signaling. A comparison of airway contraction with the ACH-induced Ca(2+) response of the SMCs revealed that the onset of airway contraction correlated with the initial Ca(2+) transient, and that sustained airway contraction correlated with the occurrence of the Ca(2+) oscillations. Buffering intracellular Ca(2+) with BAPTA prohibited Ca(2+) signaling and airway contraction, indicating a Ca(2+)-dependent pathway. Cessation of the Ca(2+) oscillations, induced by ACH-esterase, halothane, or the absence of extracellular Ca(2+) resulted in a relaxation of the airway. The concentration dependence of the airway contraction matched the concentration dependence of the increased frequency of the Ca(2+) oscillations. These results indicate that Ca(2+) oscillations, induced by ACH in murine bronchial SMCs, are generated by Ca(2+) release from the SR involving IP(3)- and ryanodine receptors, and are required to maintain airway contraction.
Figures




References
-
- Berridge, M.J., P. Lipp, and M.D. Bootman. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21. - PubMed
-
- Bootman, M.D., T.J. Collins, C.M. Peppiatt, L.S. Prothero, L. MacKenzie, P. De Smet, M. Travers, S.C. Tovey, J.T. Seo, M.J. Berridge, et al. 2001. Calcium signalling: an overview. Semin. Cell Dev. Biol. 12:3–10. - PubMed
-
- Dandurand, R.J., C.G. Wang, N.C. Phillips, and D.H. Eidelman. 1993. Responsiveness of individual airways to methacholine in adult rat lung explants. J. Appl. Physiol. 75:364–372. - PubMed
-
- Dandurand, R.J., C.G. Wang, S. Laberge, J.G. Martin, and D.H. Eidelman. 1994. In vitro allergic bronchoconstriction in the brown Norway rat. Am. J. Respir. Crit. Care Med. 149:1499–1505. - PubMed
-
- Di Virgilio, F., T.H. Steinberg, and S.C. Silverstein. 1990. Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium. 11:57–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

