Cultured rat hippocampal neural progenitors generate spontaneously active neural networks
- PMID: 11818538
- PMCID: PMC122240
- DOI: 10.1073/pnas.022646599
Cultured rat hippocampal neural progenitors generate spontaneously active neural networks
Abstract
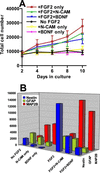
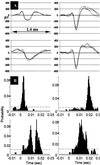
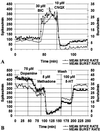
We previously demonstrated that the neural cell adhesion molecule (N-CAM) inhibited the proliferation of cultured rat hippocampal progenitor cells and increased the number of neurons generated. We demonstrate here that the continued presence of fibroblast growth factor 2 along with N-CAM or brain-derived neurotrophic factor over 12 days of culture greatly increased the number of both progenitors and neurons. These progenitor-derived neurons expressed neurotransmitters, neurotransmitter receptors, and synaptic proteins in vitro consistent with those expressed in the mature hippocampus. Progenitor cells cultured on microelectrode plates formed elaborate neural networks that exhibited spontaneously generated action potentials after 21 days. This activity was observed only in cultures grown in the presence of fibroblast growth factor 2 and either N-CAM or brain-derived neurotrophic factor. Analysis of neuronal activity after various pharmacological treatments indicated that the networks formed functional GABAergic and glutamatergic synapses. We conclude that mitogenic growth factors can synergize with N-CAM or neurotrophins to generate spontaneously active neural networks from neural progenitors.
Figures


References
-
- Palmer T D, Takahashi J, Gage F H. Mol Cell Neurosci. 1997;8:389–404. - PubMed
-
- Shetty A K, Turner D A. J Neurobiol. 1998;35:395–425. - PubMed
-
- Vicario-Abejon C, Collin C, Tsoulfas P, McKay R D. Eur J Neurosci. 2000;12:677–688. - PubMed
-
- Sah D W, Ray J, Gage F H. J Neurobiol. 1997;32:95–110. - PubMed
-
- Tanaka T, Saito H, Matsuki N. Brain Res. 1996;723:190–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

