Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex
- PMID: 11818547
- PMCID: PMC122164
- DOI: 10.1073/pnas.032468199
Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex
Abstract
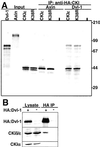
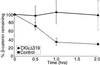
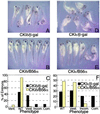
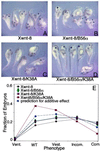
Wnt signaling plays a key role in cell proliferation and development. Recently, casein kinase I (CKI) and protein phosphatase 2A (PP2A) have emerged as positive and negative regulators of the Wnt pathway, respectively. However, it is not clear how these two enzymes with opposing functions regulate Wnt signaling. Here we show that both CKI delta and CKI epsilon interacted directly with Dvl-1, and that CKI phosphorylated multiple components of the Wnt-regulated beta-catenin degradation complex in vitro, including Dvl-1, adenomatous polyposis coli (APC), axin, and beta-catenin. Comparison of peptide maps from in vivo and in vitro phosphorylated beta-catenin and axin suggests that CKI phosphorylates these proteins in vivo as well. CKI abrogated beta-catenin degradation in Xenopus egg extracts. Notably, CKI decreased, whereas inhibition of CKI increased, the association of PP2A with the beta-catenin degradation complex in vitro. Additionally, inhibition of CKI in vivo stabilized the beta-catenin degradation complex, suggesting that CKI actively destabilizes the complex in vivo. The ability of CKI to induce secondary body axes in Xenopus embryos was reduced by the B56 regulatory subunit of PP2A, and kinase-dead CKI epsilon acted synergistically with B56 in inhibiting Wnt signaling. The data suggest that CKI phosphorylates and destabilizes the beta-catenin degradation complex, likely through the dissociation of PP2A, providing a mechanism by which CKI stabilizes beta-catenin and propagates the Wnt signal.
Figures



Similar articles
-
Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus.EMBO J. 2001 Aug 1;20(15):4122-31. doi: 10.1093/emboj/20.15.4122. EMBO J. 2001. PMID: 11483515 Free PMC article.
-
Phosphorylation and regulation of beta-catenin by casein kinase I epsilon.J Biochem. 2002 Nov;132(5):697-703. doi: 10.1093/oxfordjournals.jbchem.a003276. J Biochem. 2002. PMID: 12417018
-
Casein kinase I epsilon enhances the binding of Dvl-1 to Frat-1 and is essential for Wnt-3a-induced accumulation of beta-catenin.J Biol Chem. 2003 Apr 18;278(16):14066-73. doi: 10.1074/jbc.M213265200. Epub 2003 Jan 28. J Biol Chem. 2003. PMID: 12556519
-
New steps in the Wnt/beta-catenin signal transduction pathway.Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
-
Modulation of Wnt signaling by Axin and Axil.Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):255-65. doi: 10.1016/s1359-6101(99)00017-9. Cytokine Growth Factor Rev. 1999. PMID: 10647780 Review.
Cited by
-
Phosphorylation-Dependent Regulation of WNT/Beta-Catenin Signaling.Front Oncol. 2022 Mar 14;12:858782. doi: 10.3389/fonc.2022.858782. eCollection 2022. Front Oncol. 2022. PMID: 35359365 Free PMC article. Review.
-
Axin phosphorylation in both Wnt-off and Wnt-on states requires the tumor suppressor APC.PLoS Genet. 2018 Feb 6;14(2):e1007178. doi: 10.1371/journal.pgen.1007178. eCollection 2018 Feb. PLoS Genet. 2018. PMID: 29408853 Free PMC article.
-
The antihelmintic drug pyrvinium pamoate targets aggressive breast cancer.PLoS One. 2013 Aug 27;8(8):e71508. doi: 10.1371/journal.pone.0071508. eCollection 2013. PLoS One. 2013. PMID: 24013655 Free PMC article.
-
Association of genetic variation in genes implicated in the beta-catenin destruction complex with risk of breast cancer.Cancer Epidemiol Biomarkers Prev. 2008 Aug;17(8):2101-8. doi: 10.1158/1055-9965.EPI-08-0134. Cancer Epidemiol Biomarkers Prev. 2008. PMID: 18708403 Free PMC article.
-
Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α.Nat Chem Biol. 2010 Nov;6(11):829-36. doi: 10.1038/nchembio.453. Epub 2010 Oct 3. Nat Chem Biol. 2010. PMID: 20890287 Free PMC article.
References
-
- Bienz M, Clevers H. Cell. 2000;103:311–320. - PubMed
-
- Polakis P. Genes Dev. 2000;14:1837–1851. - PubMed
-
- Virshup D M. Curr Opin Cell Biol. 2000;12:180–185. - PubMed
-
- Seeling J M, Miller J R, Gil R, Moon R T, White R, Virshup D M. Science. 1999;283:2089–2091. - PubMed
-
- Ratcliffe M J, Itoh K, Sokol S Y. J Biol Chem. 2000;275:35680–35683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

