Leaf-to-shoot apex movement of symplastic tracer is restricted coincident with flowering in Arabidopsis
- PMID: 11818578
- PMCID: PMC122256
- DOI: 10.1073/pnas.251675698
Leaf-to-shoot apex movement of symplastic tracer is restricted coincident with flowering in Arabidopsis
Abstract
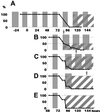
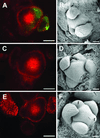
Classical experiments in plant physiology showed that leaves are the source of signals that control the development of flowers from shoot meristems. Additional physiological and genetic experiments have indicated some of the molecules (e.g., gibberellins, cytokinins, and sucrose) that promote flowering in mustards including Arabidopsis. These small hydrophilic molecules are likely to move to the shoot apex symplastically via the phloem and/or via cell-to-cell movement through plasmodesmata. To analyze potential changes in the symplastic trafficking of small molecules during the induction of flowering in Arabidopsis, we measured changes in the flow of symplastic tracers from the leaf to the shoot apex. We previously found that the onset of flowering is coincident with an evident decrease in the leaf-to-shoot trafficking of symplastic tracer molecules; this decrease in trafficking is transitory and resumes when floral development is established. Here we provide detailed analyses of symplastic connectivity during floral induction by monitoring tracer movement under different photoperiodic induction conditions and in a number of genetic backgrounds with altered flowering times. In all cases, the correlation between flowering and the reduction of symplastic tracer movement holds true. The lack of tracer movement during the induction of flowering may represent a change in plasmodesmal selectivity at this time or that a period of reduced symplastic communication is associated with floral induction.
Figures

References
-
- Chailakhyan M K. Annu Rev Plant Physiol. 1968;19:1–36.
-
- Knott J E. Proc Am Soc Hortic Sci. 1934;31:152–154.
-
- Evans L T. In: The Induction of Flowering: Some Case Histories. Evans L T, editor. New York: MacMillan; 1969. pp. 457–480.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

