Effects of hypoxia, hyperoxia on the regulation of expression and activity of matrix metalloproteinase-2 in hepatic stellate cells
- PMID: 11819847
- PMCID: PMC4695567
- DOI: 10.3748/wjg.v7.i5.647
Effects of hypoxia, hyperoxia on the regulation of expression and activity of matrix metalloproteinase-2 in hepatic stellate cells
Abstract
Aim: To study the effects of hypoxia, hyperoxia on the regulation of expression and activity of matrix metalloproteinase-2 (MMP-2) in hepatic stellate cells (HSC).
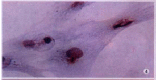
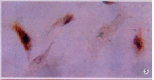
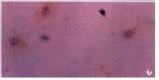
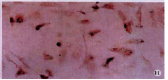
Methods: The expressions of MMP-2, tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) and membrane type matrix metalloproteinase-1 (MT1-MMP) in cultured rat HSC were detected by immunocytochemistry (ICC) and in situ hybridization (ISH). The contents of MMP-2 and TIMP-2 in culture supernatant were detected with ELISA and the activity of MMP-2 in supernatant was revealed by zymography.
Results: In the situation of hypoxia for 12h, the expression of MMP-2 protein was enhanced (hypoxia group positive indexes: 5.7 +/- 2.0, n=10; control: 3.2 +/- 1.0, n = 7; P<0.05), while TIMP-2 protein was decreased in HSC (hypoxia group positive indexes: 2.5 +/- 0.7, n = 10; control: 3.6 +/- 1.0, n = 7; P < 0.05), and the activity (total A) of MMP-2 in supernatant declined obviously (hypoxia group: 7.334 +/- 1.922, n = 9; control: 17.277 +/- 7.424, n = 11; P < 0.01). Compared the varied duration of hypoxia, the changes of expressions including mRNA and protein level as well as activity of MMP-2 were most notable in 6h group. The highest value(A(hypoxia)-A(control)) of the protein and the most intense signal of mRNA were in the period of hypoxia for 6h, along with the lowest activity of MMP-2. In the situation of hyperoxia for 12h, the contents (A(450)) of MMP-2 and TIMP-2 in supernatant were both higher than those in the control, especially the TIMP-2 (hyperoxia group: 0.0499 +/- 0.0144, n = 16; control: 0.0219 +/- 0.0098, n = 14; P < 0.01), and so was the activity of MMP-2 (hyperoxia group: 5.252 +/- 0.771, n = 14; control: 4.304 +/- 1.083, n = 12; P < 0.05), and the expression of MT1-MMP was increased.
Conclusion: HSC is sensitive to the oxygen, hypoxia enhances the expression of MMP-2 and the effect is more marked at the early stage; hyperoxia mainly raises the activity of MMP-2.
Figures







References
-
- Olaso E, Friedman SL. Molecular regulation of hepatic fibrogenesis. J Hepatol. 1998;29:836–847. - PubMed
-
- Brenner DA, Waterboer T, Choi SK, Lindquist JN, Stefanovic B, Burchardt E, Yamauchi M, Gillan A, Rippe RA. New aspects of hepatic fibrosis. J Hepatol. 2000;32:32–38. - PubMed
-
- Iredale JP, Benyon RC, Arthur MJ, Ferris WF, Alcolado R, Winwood PJ, Clark N, Murphy G. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996;24:176–184. - PubMed
-
- Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci ( Lond) 1997;92:103–112. - PubMed
-
- Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821–831. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

