Enhanced levels of cold shock proteins in Listeria monocytogenes LO28 upon exposure to low temperature and high hydrostatic pressure
- PMID: 11823178
- PMCID: PMC126669
- DOI: 10.1128/AEM.68.2.456-463.2002
Enhanced levels of cold shock proteins in Listeria monocytogenes LO28 upon exposure to low temperature and high hydrostatic pressure
Abstract
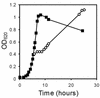
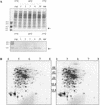
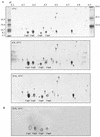
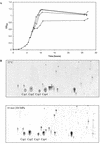
Listeria monocytogenes is a psychrotrophic food-borne pathogen that is problematic for the food industry because of its ubiquitous distribution in nature and its ability to grow at low temperatures and in the presence of high salt concentrations. Here we demonstrate that the process of adaptation to low temperature after cold shock includes elevated levels of cold shock proteins (CSPs) and that the levels of CSPs are also elevated after treatment with high hydrostatic pressure (HHP). Two-dimensional gel electrophoresis combined with Western blotting performed with anti-CspB of Bacillus subtilis was used to identify four 7-kDa proteins, designated Csp1, Csp2, Csp3, and Csp4. In addition, Southern blotting revealed four chromosomal DNA fragments that reacted with a csp probe, which also indicated that a CSP family is present in L. monocytogenes LO28. After a cold shock in which the temperature was decreased from 37 degrees C to 10 degrees C the levels of Csp1 and Csp3 increased 10- and 3.5-fold, respectively, but the levels of Csp2 and Csp4 were not elevated. Pressurization of L. monocytogenes LO28 cells resulted in 3.5- and 2-fold increases in the levels of Csp1 and Csp2, respectively. Strikingly, the level of survival after pressurization of cold-shocked cells was 100-fold higher than that of cells growing exponentially at 37 degrees C. These findings imply that cold-shocked cells are protected from HHP treatment, which may affect the efficiency of combined preservation techniques.
Figures

References
-
- Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

