Characterization and application of xylene monooxygenase for multistep biocatalysis
- PMID: 11823191
- PMCID: PMC126720
- DOI: 10.1128/AEM.68.2.560-568.2002
Characterization and application of xylene monooxygenase for multistep biocatalysis
Abstract
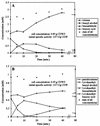
Xylene monooxygenase of Pseudomonas putida mt-2 catalyzes multistep oxidations of one methyl group of toluene and xylenes. Recombinant Escherichia coli expressing the monooxygenase genes xylM and xylA catalyzes the oxygenation of toluene, pseudocumene, the corresponding alcohols, and the corresponding aldehydes, all by a monooxygenation type of reaction (B. Bühler, A. Schmid, B. Hauer, and B. Witholt, J. Biol. Chem. 275:10085-10092, 2000). Using E. coli expressing xylMA, we investigated the kinetics of this one-enzyme three-step biotransformation. We found that unoxidized substrates like toluene and pseudocumene inhibit the second and third oxygenation steps and that the corresponding alcohols inhibit the third oxygenation step. These inhibitions might promote the energetically more favorable alcohol and aldehyde dehydrogenations in the wild type. Growth of E. coli was strongly affected by low concentrations of pseudocumene and its products. Toxicity and solubility problems were overcome by the use of a two-liquid-phase system with bis(2-ethylhexyl)phthalate as the carrier solvent, allowing high overall substrate and product concentrations. In a fed-batch-based two-liquid-phase process with pseudocumene as the substrate, we observed the consecutive accumulation of aldehyde, acid, and alcohol. Our results indicate that, depending on the reaction conditions, product formation could be directed to one specific product.
Figures





References
-
- Bell-Parikh, L. C., and F. P. Guengerich. 1999. Kinetics of cytochrome P450 2E1-catalyzed oxidation of ethanol to acetic acid via acetaldehyde. J. Biol. Chem. 274:23833-23840. - PubMed
-
- Bühler, B., A. Schmid, B. Hauer, and B. Witholt. 2000. Xylene monooxygenase catalyzes the multistep oxygenation of toluene and pseudocumene to corresponding alcohols, aldehydes, and acids in Escherichia coli JM101. J. Biol. Chem. 275:10085-10092. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

