Identical ring cleavage products during anaerobic degradation of naphthalene, 2-methylnaphthalene, and tetralin indicate a new metabolic pathway
- PMID: 11823228
- PMCID: PMC126706
- DOI: 10.1128/AEM.68.2.852-858.2002
Identical ring cleavage products during anaerobic degradation of naphthalene, 2-methylnaphthalene, and tetralin indicate a new metabolic pathway
Abstract
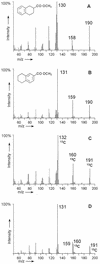
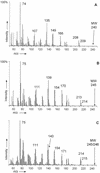
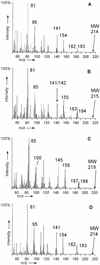
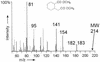
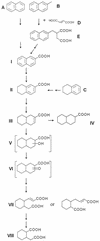
Anaerobic degradation of naphthalene, 2-methylnaphthalene, and tetralin (1,2,3,4-tetrahydronaphthalene) was investigated with a sulfate-reducing enrichment culture obtained from a contaminated aquifer. Degradation studies with tetralin revealed 5,6,7,8-tetrahydro-2-naphthoic acid as a major metabolite indicating activation by addition of a C(1) unit to tetralin, comparable to the formation of 2-naphthoic acid in anaerobic naphthalene degradation. The activation reaction was specific for the aromatic ring of tetralin; 1,2,3,4-tetrahydro-2-naphthoic acid was not detected. The reduced 2-naphthoic acid derivatives tetrahydro-, octahydro-, and decahydro-2-naphthoic acid were identified consistently in supernatants of cultures grown with either naphthalene, 2-methylnaphthalene, or tetralin. In addition, two common ring cleavage products were identified. Gas chromatography-mass spectrometry (GC-MS) and high-resolution GC-MS analyses revealed a compound with a cyclohexane ring and two carboxylic acid side chains as one of the first ring cleavage products. The elemental composition was C(11)H(16)O(4) (C(11)H(16)O(4)-diacid), indicating that all carbon atoms of the precursor 2-naphthoic acid structure were preserved in this ring cleavage product. According to the mass spectrum, the side chains could be either an acetic acid and a propenic acid, or a carboxy group and a butenic acid side chain. A further ring cleavage product was identified as 2-carboxycyclohexylacetic acid and was assumed to be formed by beta-oxidation of one of the side chains of the C(11)H(16)O(4)-diacid. Stable isotope-labeling growth experiments with either (13)C-labeled naphthalene, per-deuterated naphthalene-d(8), or a (13)C-bicarbonate-buffered medium showed that the ring cleavage products derived from the introduced carbon source naphthalene. The series of identified metabolites suggests that anaerobic degradation of naphthalenes proceeds via reduction of the aromatic ring system of 2-naphthoic acid to initiate ring cleavage in analogy to the benzoyl-coenzyme A pathway for monoaromatic hydrocarbons. Our findings provide strong indications that further degradation goes through saturated compounds with a cyclohexane ring structure and not through monoaromatic compounds. A metabolic pathway for anaerobic degradation of bicyclic aromatic hydrocarbons with 2-naphthoic acid as the central intermediate is proposed.
Figures
References
-
- Bedessem, M. E., N. G. Swoboda-Colberg, and P. J. S. Colberg. 1997. Naphthalene mineralization coupled to sulfate-reduction in aquifer-derived enrichments. FEMS Microbiol. Lett. 152:213-218.
-
- Biegert, T., G. Fuchs, and J. Heider. 1996. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur. J. Biochem. 238:661-668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

