Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration
- PMID: 11823413
- PMCID: PMC125833
- DOI: 10.1093/emboj/21.3.202
Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration
Erratum in
- EMBO J 2002 Mar 1;21(5):1240
Abstract
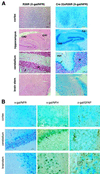
Prion protein (PrP) plays a crucial role in prion disease, but its physiological function remains unclear. Mice with gene deletions restricted to the coding region of PrP have only minor phenotypic deficits, but are resistant to prion disease. We generated double transgenic mice using the Cre-loxP system to examine the effects of PrP depletion on neuronal survival and function in adult brain. Cre-mediated ablation of PrP in neurons occurred after 9 weeks. We found that the mice remained healthy without evidence of neurodegeneration or other histopathological changes for up to 15 months post-knockout. However, on neurophysiological evaluation, they showed significant reduction of afterhyperpolarization potentials (AHPs) in hippocampal CA1 cells, suggesting a direct role for PrP in the modulation of neuronal excitability. These data provide new insights into PrP function. Furthermore, they show that acute depletion of PrP does not affect neuronal survival in this model, ruling out loss of PrP function as a pathogenic mechanism in prion disease and validating therapeutic approaches targeting PrP.
Figures





References
-
- Barrow P.A., Holmgren,C.D., Tapper,A.J. and Jeffreys,J.G.R. (1999) Intrinsic physiological and morphological properties of principal cells of the hippocampus and neocortex in hamsters infected with scrapie. Neurobiol. Dis., 6, 406–423. - PubMed
-
- Barrow P.A., Mallucci,G.R., Collinge,J. and Jefferys,J.G.R. (2000) Rescue of a physiological phenotype in mice with ablated PrP gene but expressing a transgene cassette for PrP. J. Physiol., 523P, 203P (Abstract).
-
- Brandner S., Isenmann,S., Raeber,A., Fischer,M., Sailer,A., Kobayashi,Y., Marino,S., Weissmann,C. and Aguzzi,A. (1996) Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature, 379, 339–343. - PubMed
-
- Brown D.R. et al. (1997a) The cellular prion protein binds copper in vivo. Nature, 390, 684–687. - PubMed
-
- Brown D.R., Schulz-Schaeffer,W.J., Schmidt,B. and Kretzschmar,H.A. (1997b) Prion protein-deficient cells show altered response to oxidative stress due to decreased SOD-1 activity. Exp. Neurol., 146, 104–112. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

