The Vtc proteins in vacuole fusion: coupling NSF activity to V(0) trans-complex formation
- PMID: 11823419
- PMCID: PMC125839
- DOI: 10.1093/emboj/21.3.259
The Vtc proteins in vacuole fusion: coupling NSF activity to V(0) trans-complex formation
Abstract
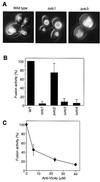
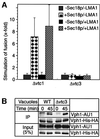
The fusion of cellular membranes comprises several steps; membrane attachment requires priming of SNAREs and tethering factors by Sec18p/NSF (N-ethylmaleimide sensitive factor) and LMA1. This leads to trans-SNARE pairing, i.e. formation of SNARE complexes between apposed membranes. The yeast vacuole system has revealed two subsequent molecular events: trans-complex formation of V-ATPase proteolipid sectors (V(0)) and release of LMA1 from the membrane. We have now identified a hetero-oligomeric membrane integral complex of vacuolar transporter chaperone (Vtc) proteins integrating these events. The Vtc complex associates with the R-SNARE Nyv1p and with V(0). Subunits Vtc1p and Vtc4p control the initial steps of fusion. They are required for Sec18p/NSF activity in SNARE priming, membrane binding of LMA1 and V(0) trans-complex formation. In contrast, subunit Vtc3p is required for the latest step, LMA1 release, but dispensible for all preceding steps, including V(0) trans-complex formation. This suggests that Vtc3p might act close to or at fusion pore opening. We propose that Vtc proteins may couple ATP-dependent NSF activity to a subset of V(0) sectors in order to activate them for V(0) trans-complex formation and/or control fusion pore opening.
Figures





References
-
- Brachmann C.B., Davies,A., Cost,G.J., Caputo,E., Li,J., Hieter,P. and Boeke,J.D. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast, 14, 115–132. - PubMed
-
- Chen Y.A., Scales,S.J., Patel,S.M., Doung,Y.-C. and Scheller,R.H. (1999) SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell, 97, 165–174. - PubMed
-
- Cohen A., Perzov,N., Nelson,H. and Nelson,N. (1999) A novel family of yeast chaperones involved in the distribution of V-ATPase and other membrane proteins. J. Biol. Chem., 274, 26885–26893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

