Importins fulfil a dual function as nuclear import receptors and cytoplasmic chaperones for exposed basic domains
- PMID: 11823430
- PMCID: PMC125346
- DOI: 10.1093/emboj/21.3.377
Importins fulfil a dual function as nuclear import receptors and cytoplasmic chaperones for exposed basic domains
Abstract
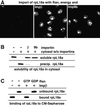
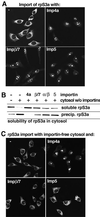
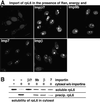
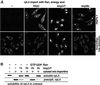
Many nuclear transport pathways are mediated by importin beta-related transport receptors. Here, we identify human importin (Imp) 4b as well as mouse Imp4a, Imp9a and Imp9b as novel family members. Imp4a mediates import of the ribosomal protein (rp) S3a, while Imp9a and Imp9b import rpS7, rpL18a and apparently numerous other substrates. Ribosomal proteins, histones and many other nuclear import substrates are very basic proteins that aggregate easily with cytoplasmic polyanions such as RNA. Imp9 effectively prevents such precipitation of, for example, rpS7 and rpL18a by covering their basic domains. The same applies to Imp4, Imp5, Imp7 and Impbeta and their respective basic import substrates. The Impbeta-Imp7 heterodimer appears specialized for the most basic proteins, such as rpL4, rpL6 and histone H1, and is necessary and sufficient to keep them soluble in a cytoplasmic environment prior to rRNA or DNA binding, respectively. Thus, just as heat shock proteins function as chaperones for exposed hydrophobic patches, importins act as chaperones for exposed basic domains, and we suggest that this represents a major and general cellular function of importins.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

