Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity
- PMID: 11825874
- PMCID: PMC155336
- DOI: 10.1101/gad.940902
Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity
Abstract
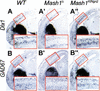
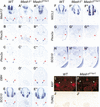
The neural bHLH genes Mash1 and Ngn2 are expressed in complementary populations of neural progenitors in the central and peripheral nervous systems. Here, we have systematically compared the activities of the two genes during neural development by generating replacement mutations in mice in which the coding sequences of Mash1 and Ngn2 were swapped. Using this approach, we demonstrate that Mash1 has the capacity to respecify the identity of neuronal populations normally derived from Ngn2-expressing progenitors in the dorsal telencephalon and ventral spinal cord. In contrast, misexpression of Ngn2 in Mash1-expressing progenitors does not result in any overt change in neuronal phenotype. Taken together, these results demonstrate that Mash1 and Ngn2 have divergent functions in specification of neuronal subtype identity, with Mash1 having the characteristics of an instructive determinant whereas Ngn2 functions as a permissive factor that must act in combination with other factors to specify neuronal phenotypes. Moreover, the ectopic expression of Ngn2 can rescue the neurogenesis defects of Mash1 null mutants in the ventral telencephalon and sympathetic ganglia but not in the ventral spinal cord and the locus coeruleus, indicating that Mash1 contribution to the specification of neuronal fates varies greatly in different lineages, presumably depending on the presence of other determinants of neuronal identity.
Figures






References
-
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19:27–37. - PubMed
-
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. - PubMed
-
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. - PubMed
-
- Bray S. Specificity and promiscuity among proneural proteins. Neuron. 2000;25:1–2. - PubMed
-
- Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
