Role of picornaviruses in flu-like illnesses of adults enrolled in an oseltamivir treatment study who had no evidence of influenza virus infection
- PMID: 11825938
- PMCID: PMC153349
- DOI: 10.1128/JCM.40.2.330-334.2002
Role of picornaviruses in flu-like illnesses of adults enrolled in an oseltamivir treatment study who had no evidence of influenza virus infection
Abstract
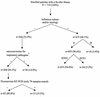
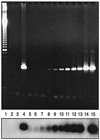
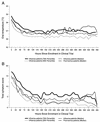
The primary objective of this study was to determine the role of picornavirus in flu-like episodes (temperature of > or =38.0 degrees C plus one respiratory and one constitutional symptom) among otherwise healthy adults enrolled in a placebo-controlled, double-blind, randomized oseltamivir treatment study. Combined nasal and pharyngeal swabs were collected at baseline for influenza cultures and picornavirus reverse transcription (RT)-PCR. In addition, acute- and convalescent-serum samples were obtained for serological studies of common respiratory pathogens. From a total of 719 subjects enrolled in the clinical trial within 36 h of the onset of symptoms, 475 (66%) had evidence of recent influenza A or B virus infections by means of culture and/or serological testing. Of the 244 remaining patients, 36 (15%) presented a seroconversion for at least one of the common respiratory viruses or atypical pathogens. An RT-PCR assay for the picornavirus 5" noncoding region (NCR) was positive in a subset of 15 (19%) of 78 patients with flu-like illnesses of undetermined etiology. Sequence analysis of the picornavirus 5" NCR amplicons revealed that 14 (93%) of them had greater homology to rhinoviruses, whereas 1 (7%) was related to enteroviruses. Interestingly, median total symptom scores and oral temperatures of picornavirus-positive patients (n = 15) and placebo-treated influenza virus-positive patients (n = 161) were similar over a 3-week period. We conclude that, among the influenza virus-negative preselected cases of this study, rhinoviruses were relatively frequent pathogens associated with important respiratory and systemic symptoms.
Figures
References
-
- Boivin, G., I. Hardy, G. Tellier, and J. Maziade. 2000. Predicting influenza infections during epidemics with use of a clinical case definition. Clin. Infect. Dis. 31:1166-1169. - PubMed
-
- Brandenburg, A. H., J. Groen, H. A. van Steensel-Moll, E. C. Claas, P. H. Rothbarth, H. J. Neijens, and A. D. Osterhaus. 1997. Respiratory syncytial virus specific serum antibodies in infants under six months of age: limited serological response upon infection. J. Med. Virol. 52:97-104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

