Process outgrowth of oligodendrocytes is promoted by interaction of fyn kinase with the cytoskeletal protein tau
- PMID: 11826099
- PMCID: PMC6758498
- DOI: 10.1523/JNEUROSCI.22-03-00698.2002
Process outgrowth of oligodendrocytes is promoted by interaction of fyn kinase with the cytoskeletal protein tau
Abstract
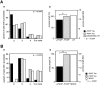
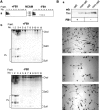
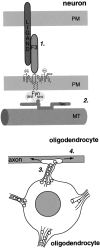
Fyn kinase plays an important role during myelination and has been shown to promote morphological differentiation of cultured oligodendrocytes. We analyzed the downstream targets of Fyn kinase in oligodendrocytes. Because process outgrowth and wrapping of axons involve cytoskeletal rearrangement, we focused on cytoskeletal proteins linked to Fyn. Here we demonstrate that Fyn binds to the cytoskeletal proteins Tau and alpha-Tubulin in oligodendrocytes. Tau interacts with the Fyn SH3 domain whereas alpha-Tubulin binds to the Fyn SH2 and SH3 domains. To study the function of the Fyn-Tau interaction in oligodendrocytes, we designed a Tau deletion mutant that would compete with endogenous Tau-Fyn binding in transfected cells. The mutant Tau protein binds to the Fyn SH3 domain but lacks the microtubuli interaction domain and thus cannot bind to microtubuli. In the presence of the mutant Tau protein, a reduction of the process number and process length in oligodendroglial cells was observed. This effect is likely to be caused by interference with the Fyn-Tau-microtubuli cascade rather than inactivation of the kinase, because Fyn bound to the mutant Tau retains activity. A similar inhibition of process outgrowth was observed when oliogodendroglial cells were cultured in the presence of Fumonisin B1, an inhibitor of sphingolipid synthesis that prevents the formation of rafts. Because ligation of the cell adhesion molecule F3 on oligodendrocytes leads to activation of Fyn kinase localized in rafts, these findings suggest that recruitment of Tau and Tubulin to activated Fyn kinase in rafts is an important step in the initiation of myelination.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
