Ultrastructure of a somatic spine mat for nicotinic signaling in neurons
- PMID: 11826104
- PMCID: PMC6758519
- DOI: 10.1523/JNEUROSCI.22-03-00748.2002
Ultrastructure of a somatic spine mat for nicotinic signaling in neurons
Abstract
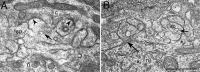
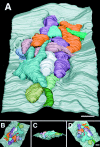
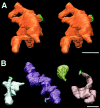
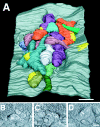
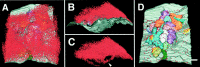
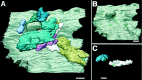
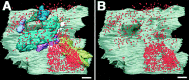
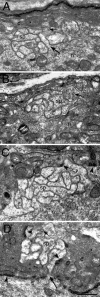
Chick ciliary neurons have somatic spines grouped in discrete clumps or mats tightly folded against the soma and enriched in nicotinic receptors containing alpha7 subunits. An embryonic ciliary neuron has one to two dozen such spine mats, all overlaid by a large presynaptic calyx engulfing the cell. Three-dimensional tomographic reconstruction from serial thick sections revealed 13 somatic spines in one complete spine mat on a ciliary neuron late in embryogenesis. The spines varied in morphology and usually were branched but had numerous similarities to dendritic spines, including mean length, volume, surface area, presence of endoplasmic reticulum, and occasional multivesicular bodies. The spines invariably were connected to the soma via a narrow neck of approximately 0.2 micrometer in diameter as found for dendritic spines, suggesting restricted access from spine lumen to soma. A prominent difference between dendritic and somatic spines is the absence of postsynaptic densities from most somatic spines both on embryonic and adult ciliary neurons. Transmitter access to receptors on the spines may occur either by lateral diffusion from release sites over nearby postsynaptic densities or by release directly onto spines from the overlying calyx lined with vesicles. The latter is less likely in the adult, where some spines are adjacent to but not overlaid by vesicle-enriched presynaptic structures. The anatomical configuration of spine mats suggests coordinate spine activation by transmitter release into a confined volume while spine morphology is used to control the chemical consequences of synaptic signaling.
Figures


References
-
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. - PubMed
-
- Alkondon M, Pereira EFR, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of α7 nicotinic acetylcholine receptors in rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. - PubMed
-
- Bundman MC, Pico RM, Gall CM. Ultrastructural plasticity of the dentate gyrus granule cells following recurrent limbic seizures. I. Increase in somatic spines. Hippocampus. 1994;4:601–610. - PubMed
-
- Chang K, Berg DK. Voltage-gated channels block nicotinic regulation of CREB phosphorylation and gene expression in neurons. Neuron. 2001;32:855–865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
