Repellent guidance of regenerating optic axons by chondroitin sulfate glycosaminoglycans in zebrafish
- PMID: 11826114
- PMCID: PMC6758477
- DOI: 10.1523/JNEUROSCI.22-03-00842.2002
Repellent guidance of regenerating optic axons by chondroitin sulfate glycosaminoglycans in zebrafish
Abstract
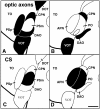
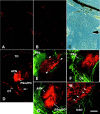
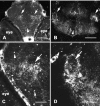
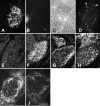
We analyzed the role of chondroitin sulfate (CS) glycosaminoglycans, putative inhibitors of axonal regeneration in mammals, in the regenerating visual pathway of adult zebrafish. In the adult, CS immunoreactivity was not detectable before or after an optic nerve crush in the optic nerve and tract but was constitutively present in developing and adult nonretinorecipient pretectal brain nuclei, where CSs may form a boundary preventing regenerating optic fibers from growing into these inappropriate locations. Enzymatic removal of CSs by chondroitinase ABC after optic nerve crush significantly increased the number of animals showing erroneous growth of optic axons into the nonretinorecipient magnocellular superficial/posterior pretectal nucleus (83% vs 42% in controls). In vitro, a substrate border of CSs, but not heparan sulfates, strongly repelled regenerating retinal axons from adult zebrafish. We conclude that CSs contribute to repellent axon guidance during regeneration of the optic projection in zebrafish.
Figures


References
-
- Anderson RB, Walz A, Holt CE, Key B. Chondroitin sulfates modulate axon guidance in embryonic Xenopus brain. Dev Biol. 1998;202:235–243. - PubMed
-
- Avnur Z, Geiger B. Immunocytochemical localization of native chondroitin-sulfate in tissues and cultured cells using specific monoclonal antibodies. Cell. 1984;38:811–822. - PubMed
-
- Battisti WP, Shinar Y, Schwartz M, Levitt P, Murray M. Temporal and spatial patterns of expression of laminin, chondroitin sulphate proteoglycan and HNK-1 immunoreactivity during regeneration in the goldfish optic nerve. J Neurocytol. 1992;21:557–573. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
