Spatial buffering during slow and paroxysmal sleep oscillations in cortical networks of glial cells in vivo
- PMID: 11826133
- PMCID: PMC6758489
- DOI: 10.1523/JNEUROSCI.22-03-01042.2002
Spatial buffering during slow and paroxysmal sleep oscillations in cortical networks of glial cells in vivo
Abstract
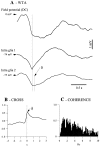
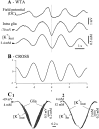
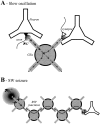
The ability of neuroglia to buffer local increases of extracellular K(+) has been known from in vitro studies. This property may confer on these cells an active role in the modulation and spreading of cortical oscillatory activities. We addressed the question of the spatial buffering in vivo by performing single and double intraglial recordings, together with measures of the extracellular K(+) and Ca(2+) concentrations ([K(+)](out) and [Ca(2+)](out)) in the cerebral cortex of cats under ketamine and xylazine anesthesia during patterns of slow sleep oscillations and spike-wave seizures. In addition, we estimated the fluctuations of intraglial K(+) concentrations ([K(+)](in)). Measurements obtained during the slow oscillation indicated that glial cells phasically take up part of the extracellular K(+) extruded by neurons during the depolarizing phase of the slow oscillation. During this condition, the redistribution of K(+) appeared to be local. Large steady increases of [K(+)](out) and phasic potassium accumulations were measured during spike-wave seizures. In this condition, [K(+)](in) rose before [K(+)](out) if the glial cells were located at some distance from the epileptic focus, suggesting faster K(+) diffusion through the interglial syncytium. The simultaneously recorded [Ca(2+)](out) dropped steadily during the seizures to levels incompatible with efficient synaptic transmission, but also displayed periodic oscillations, in phase with the intraseizure spike-wave complexes. In view of this fact, and considering the capability of K(+) to modulate neuronal excitability both at the presynaptic and postsynaptic levels, we suggest that the K(+) long-range spatial buffering operated by glia is a parallel synchronizing and/or spreading mechanism during paroxysmal oscillations.
Figures








References
-
- Achermann P, Borbély AA. Low-frequency (<1 Hz) oscillations in the human sleep EEG. Neuroscience. 1997;81:213–222. - PubMed
-
- Alici K, Heinemann U. Effects of low glucose levels on changes in [Ca2+]o induced by stimulation of Schaffer collaterals under conditions of blocked chemical synaptic transmission in rat hippocampal slices. Neurosci Lett. 1995;185:5–8. - PubMed
-
- Ammann D. Ion selective microelectrodes. Springer; Berlin: 1986.
-
- Amzica F, Neckelmann D. Membrane capacitance of cortical neurons and glia during sleep oscillations and spike-wave seizures. J Neurophysiol. 1999;82:2731–2746. - PubMed
-
- Amzica F, Steriade M. The K-complex: its slow (<1 Hz) rhythmicity and relation to delta waves. Neurology. 1997;49:952–959. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
