Persistent behavioral sensitization to chronic L-DOPA requires A2A adenosine receptors
- PMID: 11826134
- PMCID: PMC6758487
- DOI: 10.1523/JNEUROSCI.22-03-01054.2002
Persistent behavioral sensitization to chronic L-DOPA requires A2A adenosine receptors
Abstract
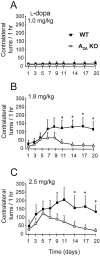
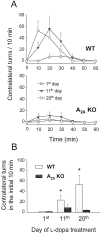
To investigate the role of A(2A) adenosine receptors in adaptive responses to chronic intermittent dopamine receptor stimulation, we compared the behavioral sensitization elicited by repeated l-DOPA treatment in hemiparkinsonian wild-type (WT) and A(2A) adenosine receptor knock-out (A(2A) KO) mice. Although the unilateral nigrostriatal lesion produced by intrastriatal injection of 6-hydroxydopamine was indistinguishable between WT and A(2A) KO mice, they developed strikingly different patterns of behavioral sensitization after daily treatment with low doses of l-DOPA for 3 weeks. WT mice initially displayed modest contralateral rotational responses and then developed progressively greater responses that reached a maximum within 1 week and persisted for the duration of the treatment. In contrast, any rotational behavioral sensitization in A(2A) KO mice was transient and completely reversed within 2 weeks. Similarly, the time to reach the peak rotation was progressively shortened in WT mice but remained unchanged in A(2A) KO mice. Furthermore, daily l-DOPA treatment produced gradually sensitized grooming in WT mice but failed to induce any sensitized grooming in A(2A) KO mice. Finally, repeated l-DOPA treatment reversed the 6-OHDA-induced reduction of striatal dynorphin mRNA in WT but not A(2A) KO mice, raising the possibility that the A(2A) receptor may contribute to l-DOPA-induced behavioral sensitization by facilitating adaptations within the dynorphin-expressing striatonigral pathway. Together these results demonstrate that the A(2A) receptor plays a critical role in the development and particularly the persistence of behavioral sensitization to repeated l-DOPA treatment. Furthermore, they raise the possibility that the maladaptive dyskinetic responses to chronic l-DOPA treatment in Parkinson's disease may be attenuated by A(2A) receptor inactivation.
Figures


References
-
- Andersson M, Hilbertson A, Cenci Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol Dis. 1999;6:461–474. - PubMed
-
- Barone P, Morelli M, Popoli M, Ciccarelli G, Campanella G, Di Chiara G. Behavioural sensitization in 6-hydroxydopamine lesioned rats involves the dopamine signal transduction: changes in DARPP-32 phosphorylation. Neuroscience. 1994;61:867–873. - PubMed
-
- Blanchet PJ, Gomez-Mancilla B, Bedard PJ. DOPA-induced “peak dose” dyskinesia: clues implicating D2 receptor-mediated mechanisms using dopaminergic agonists in MPTP monkeys. J Neural Transm Suppl. 1995;45:103–112. - PubMed
-
- Bordet R, Ridray S, Schwartz JC, Sokoloff P. Involvement of the direct striatonigral pathway in levodopa-induced sensitization in 6-hydroxydopamine-lesioned rats. Eur J Neurosci. 2000;2:2117–2123. - PubMed
-
- Brotchie JM. Adjuncts to dopamine replacement: a pragmatic approach to reducing the problem of dyskinesia in Parkinson's disease. Mov Disord. 1998;13:871–876. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
