A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes
- PMID: 11826160
- PMCID: PMC2290110
- DOI: 10.1113/jphysiol.2001.013101
A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes
Abstract
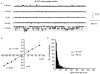
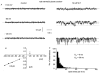
In vascular smooth muscle cells many agonists cause the release of Ca2+ ions from internal stores. An important problem concerns the mechanism by which the intracellular stores are refilled subsequent to depletion. In the present study, we describe the properties of a Ca2+-permeable non-selective cation channel current that is activated in rabbit portal vein myocytes by depletion of internal Ca2+ stores. Application of cyclopiazonic acid (CPA), which depletes internal Ca2+ stores, activated whole-cell currents that had a reversal potential (E(r)) of about +50 mV in 1.5 mM external Ca2+ (Ca2+o). In 0 mM Ca2+o, the currents were larger and E(r) was approximately 0 mV. Application of CPA and caffeine during cell-attached recording activated single inward channel currents at negative potentials, which had a slope conductance of 2-3 pS and an E(r) of +20 mV. The slope conductance in 0 and 110 mM Ca2+o was 7 and 1.5 pS, respectively, and E(r) values indicated that these non-selective cation channels are highly permeable to Ca2+ ions. Bath application of the cell-permeant Ca2+ chelator, BAPTA-AM, also activated similar currents, indicating that these channels are not activated by Ca2+. Spontaneous channel currents with similar properties to store-operated channels were observed in some patches. Application of W-7, an inhibitor of the Ca2+-binding protein calmodulin, also activated similar Ca2+-permeable channel currents. In conclusion, it is demonstrated that agents that deplete Ca2+ stores and inhibit calmodulin binding activate Ca2+-permeable non-selective cation channel currents in rabbit portal vein myocytes. These channels may have an important role in vascular smooth muscle in providing an influx of Ca2+ to refill depleted internal Ca2+ stores and appear to possess different characteristics to store-operated channels described in other vascular smooth muscle preparations.
Figures





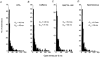
References
-
- Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX-J. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. American Journal of Physiology - Heart and Circulatory Physiology. 2001;H280:746–755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

