Propionate-induced relaxation in rat mesenteric arteries: a role for endothelium-derived hyperpolarising factor
- PMID: 11826171
- PMCID: PMC2290101
- DOI: 10.1113/jphysiol.2001.013105
Propionate-induced relaxation in rat mesenteric arteries: a role for endothelium-derived hyperpolarising factor
Abstract
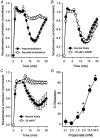
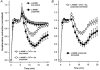
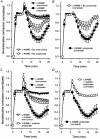
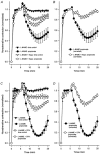
Short chain fatty acids, including propionate, are generated in the caecum and large intestine, and when absorbed may elicit localised increases in intestinal blood flow. We sought to assess the mechanism by which propionate caused vasorelaxation. Propionate-mediated relaxation of noradrenaline-preconstricted rat mesenteric small arteries (RMSAs, i.d. 200-300 microm) was studied using small vessel myography. Propionate (1-30 mM) produced a concentration-dependent relaxation. Relaxation induced by 10 mM propionate (the approximate EC50) was almost abolished by endothelial denudation, although a marked relaxation to a very high concentration of propionate (50 mM) persisted in the absence of the endothelium. In endothelium-intact RMSAs, relaxation to 10 mM propionate was almost abolished by elevating [K+]o to 25 mM, but was unaffected by 100 microM N(omega)-nitro-L-arginine methyl ester (L-NAME) (68 +/- 4 vs. 66 +/- 3% in controls, n = 35), or by 1 microM indomethacin (60 +/- 4 vs. 61 +/- 7 % in controls, n = 15). In the presence of L-NAME, relaxation to 10 mM propionate was significantly and markedly (i.e. > 50 %) inhibited by 50 microM Ba2+ and by the combination of 100 nM charybdotoxin and 100 nM apamin. A similar effect on propionate-mediated relaxation was also exerted by 100 microM ouabain, and by the combination of 50 microM barium with ouabain. Relaxation was also significantly and markedly inhibited by pre-treatment of RMSAs with 100 nM thapsigargin or 10 microM cyclopiazonic acid (CPA). The results demonstrate that 10 mM propionate relaxes RMSAs via endothelium-derived hyperpolarising factor (EDHF). The observation that relaxation by propionate is inhibited by thapsigargin and CPA suggests that this action of propionate involves the release of endothelial cell Ca2+ stores.
Figures





References
-
- Aalkjaer C, Poston L. Effects of pH on vascular tension: which are the important mechanisms? Journal of Vascular Research. 1996;33:347–359. - PubMed
-
- Austin C, Wray S. A quantitative study of the relation between intracellular pH and force in rat mesenteric vascular smooth muscle. Pflugers Archiv. 1994;427:270–276. - PubMed
-
- Austin C, Wray S. Interactions between Ca2+ and H+ and functional consequences in vascular smooth muscle. Circulation Research. 2000;86:355–363. - PubMed
-
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identitifaction of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circulation Research. 1996;78:415–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

