Spinal muscular atrophy genetic testing experience at an academic medical center
- PMID: 11826188
- PMCID: PMC1906969
- DOI: 10.1016/S1525-1578(10)60680-0
Spinal muscular atrophy genetic testing experience at an academic medical center
Abstract
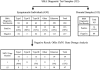
Approximately 94% of spinal muscular atrophy (SMA) patients lack both copies of SMN1 exon 7. We report our SMA genetic testing experience (total 1281 cases), using SMA linkage analysis (32 families), SMA diagnostic testing by PCR-RFLP (restriction fragment length polymorphism) to detect the homozygous absence of SMN1 exon 7 (and exon 8) (533 cases), and an assay to determine copy number of SMN1 exon 7 (SMN1 gene dosage analysis) (716 cases). SMN1 gene dosage analysis is used for SMA carrier testing as well as for the confirmation of a heterozygous SMN1 deletion in symptomatic individuals who do not lack both copies of SMN1. We conclude that comprehensive SMA testing, including SMN1 deletion analysis, SMN1 gene dosage analysis, and linkage analysis, offers the most complete evaluation of SMA patients and their families.
Figures
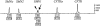
References
-
- Munsat TL, Davies KE: International SMA consortium meeting (June 26–28, 1992, Bonn, Germany). Neuromuscul Disord 1992, 2:423-428 - PubMed
-
- Zerres K, Wirth B, Rudnik-Schoeneborn S: Spinal muscular atrophy: clinical and genetic correlation. Neuromuscul Disord 1997, 7:202-207 - PubMed
-
- Clermont O, Burlet P, Burglen L, Lefebvre S, Pascal F, McPherson J, Wasmuth JJ, Cohen D, Le Paslier D, Weissenbach J, Lathrop M, Munnich A, Melki J: Use of genetic and physical mapping to locate the spinal muscular atrophy locus between two new highly polymorphic DNA markers. Am J Hum Genet 1994, 54:687-694 - PMC - PubMed
-
- Soares VM, Brzustowicz LM, Kleyn PW, Knowles JA, Palmer DA, Asokan S, Penchaszadeh GK, Munsat TL, Gilliam TC: Refinement of the spinal muscular atrophy locus to the interval between D5S435 and MAP1B. Genomics 1993, 15:365-371 - PubMed
-
- Melki J, Lefebvre S, Burglen L, Burlet P, Clermont O, Millasseau P, Reboullet S, Benichou B, Zeviani M, Le Paslier D, Cohen D, Weissenbach J, Munnich A: De novo and inherited deletions of the 5q13 region in spinal muscular atrophies. Science 1994, 264:1474-1477 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

