MR evaluation in patients with isolated anosmia since birth or early childhood
- PMID: 11827889
- PMCID: PMC7975503
MR evaluation in patients with isolated anosmia since birth or early childhood
Abstract
Background and purpose: Anosmias with chromosomal disorders has been well investigated. However, isolated anosmia (IA) has received less attention, although it occurs more frequently. We compared frontobasal structures in patients with IA since birth or early childhood with those in control subjects.
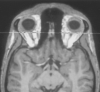
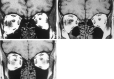
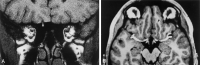
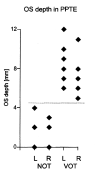
Methods: Imaging findings obtained in 16 patients with IA were compared with those obtained in eight control subjects. Imaging was performed with a standard quadrature head coil at 1.5 T. T1-weighted spin-echo (coronal plane perpendicular to frontal skull base; section thickness, 3 mm; pixels, 0.43 x 0.39 mm) and sagittal T1-weighted magnetization-prepared rapid gradient-echo (voxels, 1.0 x 1.0 x 1.0 mm) sequences were performed. We assessed the length and depth of the olfactory sulcus, olfactory bulb volume, and olfactory sulcus depth in the plane of the posterior tangent through the eyeballs (PPTE).
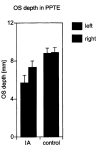
Results: Five patients with IA had bilateral hypoplastic olfactory bulbs. Three patients with IA had hypoplastic olfactory bulbs on the right and aplastic olfactory bulbs on the left. Eight patients with IA had bilaterally aplastic olfactory bulbs. The depth of the olfactory sulcus at the level of the PPTE was smaller in patients with IA than in control subjects. The depth of the olfactory sulcus was greater on the right than on the left, and there was no overlap. Among patients with IA, the depth of the olfactory sulcus differed significantly between those with and those without visible olfactory tracts.
Conclusion: The depth of the olfactory sulcus at the level of the PPTE reflects the presence of olfactory tracts. The presence or absence of the olfactory tract may therefore have some association with cortical growth of the olfactory sulcus region. The olfactory sulcus is deeper on the right than on the left, particularly in patients with IA. We speculate that olfaction may be processed predominantly in the right hemisphere.
Figures


References
-
- Kallmann FJ, Schoenfeld WA, Barrera SE. The genetic aspects of primary eunuchoidism. Am J Ment Defic 1944;48:203–236
-
- Pawlowitzki IH, Diekstall P, Schadel A, Miny P. Estimating frequency of Kallmann syndrome among hypogonadic and among anosmic patients. Am J Med Genet 1987;26:473–479 - PubMed
-
- Hazard J, Rozenberg I, Perlemuter L, et al. Gonadotropin responses to low dose pulsatile administration of GnRH in a case of anosmia with hypogonadotropic hypogonadism associated with gonadal dysgenesis 47 XXY. Acta Endocrinol 1986;113:593–597 - PubMed
-
- Layman LC. Genetics of human hypogonadotropic hypogonadism. Am J Med Genet 1999;89:240–248 - PubMed
-
- Hall JE. Physiologic and genetic insights into the pathophysiology and management of hypogonadotropic hypogonadism. Ann Endocrinol 1999;60:93–101 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
