Visualization of Rab9-mediated vesicle transport from endosomes to the trans-Golgi in living cells
- PMID: 11827983
- PMCID: PMC2173336
- DOI: 10.1083/jcb.200109030
Visualization of Rab9-mediated vesicle transport from endosomes to the trans-Golgi in living cells
Abstract
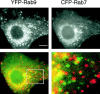
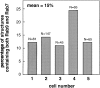
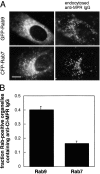
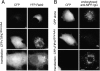
Mannose 6-phosphate receptors (MPRs) are transported from endosomes to the trans-Golgi via a transport process that requires the Rab9 GTPase and the cargo adaptor TIP47. We have generated green fluorescent protein variants of Rab9 and determined their localization in cultured cells. Rab9 is localized primarily in late endosomes and is readily distinguished from the trans-Golgi marker galactosyltransferase. Coexpression of fluorescent Rab9 and Rab7 revealed that these two late endosome Rabs occupy distinct domains within late endosome membranes. Cation-independent mannose 6-phosphate receptors are enriched in the Rab9 domain relative to the Rab7 domain. TIP47 is likely to be present in this domain because it colocalizes with the receptors in fixed cells, and a TIP47 mutant disrupted endosome morphology and sequestered MPRs intracellularly. Rab9 is present on endosomes that display bidirectional microtubule-dependent motility. Rab9-positive transport vesicles fuse with the trans-Golgi network as followed by video microscopy of live cells. These data provide the first indication that Rab9-mediated endosome to trans-Golgi transport can use a vesicle (rather than a tubular) intermediate. Our data suggest that Rab9 remains vesicle associated until docking with the Golgi complex and is rapidly removed concomitant with or just after membrane fusion.
Figures





References
-
- Carroll, K.S., J. Hanna, I. Simon, J. Krise, P. Barbero, and S.R. Pfeffer. 2001. Role of the Rab9 GTPase in facilitating receptor recruitment by TIP47. Science. 292:1373–1377. - PubMed
-
- Diaz, E., and S.R. Pfeffer. 1998. TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell. 93:433–443. - PubMed
-
- Draper, R.K., Y. Goda, F.M. Brodsky, and S.R. Pfeffer. 1990. Anti-clathrin antibodies inhibit endocytosis but not receptor recycling to the trans Golgi network in vitro. Science. 248:1539–1541. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

