Differential T cell function and fate in lymph node and nonlymphoid tissues
- PMID: 11828006
- PMCID: PMC2193599
- DOI: 10.1084/jem.20011558
Differential T cell function and fate in lymph node and nonlymphoid tissues
Abstract
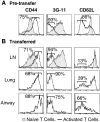
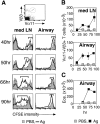
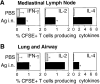
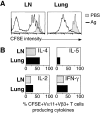
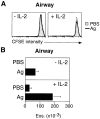
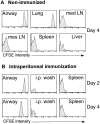
The functions and fate of antigen-experienced T cells isolated from lymph node or nonlymphoid tissues were analyzed in a system involving adoptive transfer of in vitro-activated T cells into mice. Activated T cells present in the lymph nodes could be stimulated by antigen to divide, produce effector cytokines, and migrate to peripheral tissues. By contrast, activated T cells that had migrated into nonlymphoid tissues (lung and airway) produced substantial effector cytokines upon antigen challenge, but were completely unable to divide or migrate back to the lymph nodes. Therefore, activated T cells can undergo clonal expansion in the lymph node, but are recruited and retained as nondividing cells in nonlymphoid tissues. These distinct regulatory events in lymph node and nonlymphoid tissues reveal simple key mechanisms for both inducing and limiting T cell immunity.
Figures

References
-
- Gowans, J.L., and E.J. Knight. 1964. The route of recirculation of lymphocytes in the rat. Proc. R. Soc. Lond. B. 159:257–282. - PubMed
-
- Picker, L.J., and E.C. Butcher. 1992. Physiological and molecular mechanisms of lymphocyte homing. Annu. Rev. Immunol. 10:561–581. - PubMed
-
- Koh, W.P., E. Chan, K. Scott, G. McCaughan, M. France, and B. Fazekas de St Groth. 1999. TCR-mediated involvement of CD4+ transgenic T cells in spontaneous inflammatory bowel disease in lymphopenic mice. J. Immunol. 162:7208–7216. - PubMed

