Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells
- PMID: 11828008
- PMCID: PMC2193592
- DOI: 10.1084/jem.20010934
Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells
Abstract
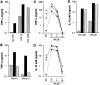
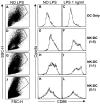
Natural killer (NK) cells and dendritic cells (DCs) are two distinct cell types of innate immunity. It is known that the in vitro interaction of human NK cells with autologous DCs results in DC lysis. Here we show that contact-dependent interactions between activated human NK cells and immature DCs (iDCs) provides a "control switch" for the immune system. At low NK/DC ratios, this interaction dramatically amplifies DC responses, whereas at high ratios it completely turns off their responses. Specifically, culture of activated human NK cells with iDCs, at low NK/DC ratios (1:5), led to exponential increases in DC cytokine production, which were completely dependent on cell-to-cell contact. DC maturation was also driven by cognate interactions with NK cells and maturation was dependent on endogenously produced TNF-alpha in the culture. At slightly higher NK/DC ratios (5:1), inhibition of DC functions was the dominant feature due to potent killing by the autologous NK cells. Resting NK cells also stimulated autologous DC maturation in a TNF-alpha/contact-dependent manner, however, increasing the NK/DC ratio only led to an enhancement of this effect.
Figures





References
-
- Banchereau, J., and R. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. - PubMed
-
- Scott, P., and G. Trinchieri. 1995. The role of natural killer cells in host-parasite interactions. Curr. Opin. Immunol. 7:34–40. - PubMed
-
- Cella, M., F. Sallusto, and A. Lanzavecchia. 1997. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 9:10–16. - PubMed

