Tail gut endoderm and gut/genitourinary/tail development: a new tissue-specific role for Hoxa13
- PMID: 11830557
- PMCID: PMC2435615
- DOI: 10.1242/dev.129.3.551
Tail gut endoderm and gut/genitourinary/tail development: a new tissue-specific role for Hoxa13
Abstract
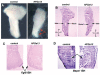
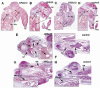
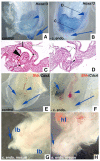
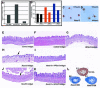
Hoxa13 is expressed early in the caudal mesoderm and endoderm of the developing hindgut. The tissue-specific roles of Hoxa13 function have not been described. Hand-foot-genital syndrome, a rare dominantly inherited human malformation syndrome characterized by distal extremity and genitourinary anomalies, is caused by mutations in the HOXA13 gene. We show evidence that one specific HOXA13 mutation likely acts as a dominant negative in vivo. When chick HFGa13 is overexpressed in the chick caudal endoderm early in development, caudal structural malformations occur. The phenotype is specific to HFGa13 expression in the posterior endoderm, and includes taillessness and severe gut/genitourinary (GGU) malformations. Finally, we show that chick HFGa13 negatively regulates expression of Hoxd13 and antagonizes functions of both endogenous Hoxa13 and Hoxd13 proteins. We suggest a fundamental role for epithelial specific expression of Hoxa13 in the epithelial-mesenchymal interaction necessary for tail growth and posterior GGU patterning.
Figures



References
-
- Akarsu AN, Stoilov I, Yilmaz E, Sayli BS, Sarfarazi M. Genomic structure of HOXD13 gene: a nine polyalanine duplication causes synpolydactyly in two unrelated families. Hum Mol Genet. 1996;5:945–52. - PubMed
-
- Beals RK, Rolfe B. VATER association. A unifying concept of multiple anomalies. J Bone Joint Surg Am. 1989;71:948–50. - PubMed
-
- Bendall AJ, Ding J, Hu G, Shen MM, Abate-Shen C. Msx1 antagonizes the myogenic activity of Pax3 in migrating limb muscle precursors. Development. 1999;126:4965–76. - PubMed
-
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

