Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability
- PMID: 11830646
- PMCID: PMC122299
- DOI: 10.1073/pnas.042688399
Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability
Abstract
Asymmetric distribution of phospholipids is ubiquitous in the plasma membranes of many eukaryotic cells. The majority of the aminophospholipids are located in the inner leaflet whereas the cholinephospholipids are localized predominantly in the outer leaflet. Several functional roles for asymmetric phospholipid distribution in plasma membranes have been suggested. Disruption of lipid asymmetry creates a procoagulant surface on platelets and serves as a trigger for macrophage recognition of apoptotic cells. Furthermore, the dynamic process of phospholipid translocation regulates important cellular events such as membrane budding and endocytosis. In the present study, we used the red cell membrane as the model system to explore the contribution of phospholipid asymmetry to the maintenance of membrane mechanical properties. We prepared two different types of membranes in terms of their phospholipid distribution, one in which phospholipids were scrambled and the other in which the asymmetric distribution of phospholipids was maintained and quantitated their mechanical properties. We documented that maintenance of asymmetric distribution of phospholipids resulted in improved membrane mechanical stability. The greater difficulty in extracting the spectrin-actin complex at low-ionic strength from the membranes with asymmetric phospholipid distribution further suggested the involvement of interactions between aminophospholipids in the inner leaflet and skeletal proteins in modulating mechanical stability of the red cell membrane. These findings have enabled us to document a functional role of lipid asymmetry in regulating membrane material properties.
Figures

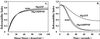


References
-
- Devaux PF. Biochemistry. 1991;30:1163–1173. - PubMed
-
- Williamson P, Schlegel R A. Mol Membr Biol. 1994;11:199–216. - PubMed
-
- Tang X, Halleck M S, Schlegel R A, Williamson P. Science. 1996;272:1495–1497. - PubMed
-
- Devaux PF. Annu Rev Biophys Biomol Struct. 1992;21:417–439. - PubMed
-
- Zwaal R F A, Schroit A J. Blood. 1997;89:1121–1132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

