The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis
- PMID: 11830673
- PMCID: PMC122241
- DOI: 10.1073/pnas.251673298
The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis
Erratum in
- Proc Natl Acad Sci U S A 2002 Apr 30;99(9):6449
Abstract
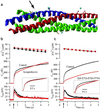
Synchronous neurotransmission depends on the tight coupling between Ca(2+) influx and fusion of neurotransmitter-filled vesicles with the plasma membrane. The vesicular soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein synaptobrevin 2 and the plasma membrane SNAREs syntaxin 1 and synaptosomal protein of 25 kDa (SNAP-25) are essential for calcium-triggered exocytosis. However, the link between calcium triggering and SNARE function remains elusive. Here we describe mutations in two sites on the surface of the SNARE complex formed by acidic and hydrophilic residues of SNAP-25 and synaptobrevin 2, which were found to coordinate divalent cations in the neuronal SNARE complex crystal structure. By reducing the net charge of the site in SNAP-25 we identify a mutation that interferes with calcium triggering of exocytosis when overexpressed in chromaffin cells. Exocytosis was elicited by photorelease of calcium from a calcium cage and evaluated by using patch-clamp capacitance measurements at millisecond time resolution. We present a method for monitoring the dependence of exocytotic rate upon calcium concentration at the release site and demonstrate that the mutation decreased the steepness of this relationship, indicating that the number of sequential calcium-binding steps preceding exocytosis is reduced by one. We conclude that the SNARE complex is linked directly to calcium triggering of exocytosis, most likely in a complex with auxiliary proteins.
Figures



References
-
- Söllner T, Whitehart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. - PubMed
-
- Jahn J, Südhof T C. Annu Rev Biochem. 1999;68:863–911. - PubMed
-
- Lin R C, Scheller R H. Annu Rev Cell Dev Biol. 2000;16:19–49. - PubMed
-
- Brose N, Petrenko A G, Südhof T C, Jahn R. Science. 1992;256:1021–1025. - PubMed
-
- Fernandez-Chacon R, Königstorfer A, Gerber S H, Garcia J, Matos M F, Stevens C F, Brose N, Rizo J, Rosenmund C, Südhof T C. Nature (London) 2001;410:41–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

