Alpha 2-adrenoceptors in the enteric nervous system: a study in alpha 2A-adrenoceptor-deficient mice
- PMID: 11834617
- PMCID: PMC1573176
- DOI: 10.1038/sj.bjp.0704512
Alpha 2-adrenoceptors in the enteric nervous system: a study in alpha 2A-adrenoceptor-deficient mice
Abstract
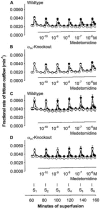
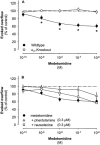
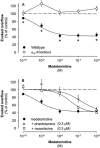
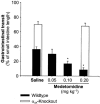
Mammals possess three types of alpha(2)-adrenoceptor, alpha(2A), alpha(2B) and alpha(2C). Our aim was to determine the type of alpha(2)-adrenoceptor involved in the control of gastrointestinal motility. In transmitter overflow experiments, myenteric plexus longitudinal muscle (MPLM) preparations of the ileum were preincubated with [(3)H]-choline and then superfused. The alpha(2)-adrenoceptor agonist medetomidine reduced the electrically evoked overflow of tritium from preparations taken from wild type but not alpha(2A)-adrenoceptor-knockout mice. In a second series of overflow experiments, MPLM preparations were preincubated with [(3)H]-noradrenaline and then superfused. Again medetomidine reduced the electrically evoked overflow of tritium from wild type but not alpha(2A)-knockout preparations. In organ bath experiments, medetomidine reduced electrically evoked contractions of segments of the ileum from wild type but not alpha(2A)-knockout mice. In each of these three series, phentolamine antagonized the effect of medetomidine in wild-type preparations with greater potency than rauwolscine. In conscious mice, gastrointestinal transit was assessed by means of an intragastric charcoal bolus. In alpha(2A)-knockout mice, the speed of gastrointestinal transit was doubled compared to wild-type. Medetomidine, injected intraperitoneally, slowed gastrointestinal transit in wild type but not alpha(2A)-knockout mice. We conclude that the cholinergic motor neurons of the enteric nervous system of mice possess alpha(2)-heteroreceptors which mediate inhibition of acetylcholine release, of neurogenic contractions and of gastrointestinal transit. The noradrenergic axons innervating the intestine possess alpha(2)-autoreceptors. Both hetero- and autoreceptors are exclusively alpha(2A). It is the alpha(2A)-adrenoceptor which in vivo mediates the inhibition of intestinal motility by the sympathetic nervous system.
Figures


Similar articles
-
All three alpha2-adrenoceptor types serve as autoreceptors in postganglionic sympathetic neurons.Naunyn Schmiedebergs Arch Pharmacol. 2003 Dec;368(6):504-12. doi: 10.1007/s00210-003-0829-x. Epub 2003 Nov 11. Naunyn Schmiedebergs Arch Pharmacol. 2003. PMID: 14610637
-
Altered prejunctional modulation of intestinal cholinergic and noradrenergic pathways by alpha2-adrenoceptors in the presence of experimental colitis.Br J Pharmacol. 2003 May;139(2):309-20. doi: 10.1038/sj.bjp.0705249. Br J Pharmacol. 2003. PMID: 12770936 Free PMC article.
-
A study of presynaptic alpha2-autoreceptors in alpha2A/D-, alpha2B- and alpha2C-adrenoceptor-deficient mice.Naunyn Schmiedebergs Arch Pharmacol. 2001 Aug;364(2):117-30. doi: 10.1007/s002100100423. Naunyn Schmiedebergs Arch Pharmacol. 2001. PMID: 11534851
-
Mechanisms of facilitation and muscarinic or alpha-adrenergic inhibition of acetylcholine and noradrenaline secretion from peripheral nerves.Acta Physiol Scand Suppl. 1982;506:1-39. Acta Physiol Scand Suppl. 1982. PMID: 6293252 Review.
-
Cannabinoids and the gastrointestinal tract.Gut. 2001 Jun;48(6):859-67. doi: 10.1136/gut.48.6.859. Gut. 2001. PMID: 11358910 Free PMC article. Review.
Cited by
-
Investigation of the distribution and function of alpha-adrenoceptors in the sheep isolated internal anal sphincter.Br J Pharmacol. 2010 Aug;160(7):1727-40. doi: 10.1111/j.1476-5381.2010.00842.x. Br J Pharmacol. 2010. PMID: 20649575 Free PMC article.
-
Sympathetic Pathways Target Cholinergic Neurons in the Human Colonic Myenteric Plexus.Front Neurosci. 2022 Mar 17;16:863662. doi: 10.3389/fnins.2022.863662. eCollection 2022. Front Neurosci. 2022. PMID: 35368277 Free PMC article.
-
Intraganglionic laminar endings and their relationships with neuronal and glial structures of myenteric ganglia in the esophagus of rat and mouse.Histochem Cell Biol. 2004 Nov;122(5):445-59. doi: 10.1007/s00418-004-0703-z. Epub 2004 Sep 18. Histochem Cell Biol. 2004. PMID: 15378379
-
A Proximal-to-Distal Survey of Healthy Adult Human Small Intestine and Colon Epithelium by Single-Cell Transcriptomics.Cell Mol Gastroenterol Hepatol. 2022;13(5):1554-1589. doi: 10.1016/j.jcmgh.2022.02.007. Epub 2022 Feb 15. Cell Mol Gastroenterol Hepatol. 2022. PMID: 35176508 Free PMC article.
-
Therapeutic strategies for colorectal cancer: antitumor efficacy of dopamine D2 receptor antagonists.Toxicol Res. 2024 Aug 7;40(4):533-540. doi: 10.1007/s43188-024-00259-8. eCollection 2024 Oct. Toxicol Res. 2024. PMID: 39345737 Review.
References
-
- ALTMAN J.D., TRENDELENBURG A.U., MACMILLAN L., BERNSTEIN D., LIMBIRD L., STARKE K., KOBILKA B.K., HEIN L. Abnormal regulation of the sympathetic nervous system in α2A-adrenergic receptor knockout mice. Mol. Pharmacol. 1999;56:154–161. - PubMed
-
- BERLIOZ F., MAORET J.J., PARIS H., LABURTHE M., FARINOTTI R., ROZÉ C. α2-Adrenergic receptors stimulate oligopeptide transport in a human intestinal cell line. J. Pharmacol. Exp. Ther. 2000;294:466–472. - PubMed
-
- BLANDIZZI C., DODA M., TARKOVÁCS G., DEL TACCA M., VIZI E.S. Functional evidence that acetylcholine release from Auerbach's plexus of guinea-pig ileum is modulated by α2A-adrenoceptor subtype. Eur. J. Pharmacol. 1991;205:311–313. - PubMed
-
- BLANDIZZI C., TARKOVÁCS G., NATALE G., DEL TACCA M., VIZI E.S. Functional evidence that [3H] acetylcholine and [3H] noradrenaline release from guinea pig ileal myenteric plexus and noradrenergic terminals is modulated by different presynaptic α2-adrenoceptor subtypes. J. Pharmacol. Exp. Ther. 1993;267:1054–1060. - PubMed
-
- BÜCHELER M., LOHSE M.J., HEIN L. Two presynaptic α2-adrenergic receptor subtypes are required for regulation of dopamine release in mouse basal ganglia. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:R26.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

