Efficient cleavage of ribosome-associated poly(A)-binding protein by enterovirus 3C protease
- PMID: 11836384
- PMCID: PMC135927
- DOI: 10.1128/jvi.76.5.2062-2074.2002
Efficient cleavage of ribosome-associated poly(A)-binding protein by enterovirus 3C protease
Abstract
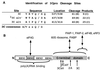
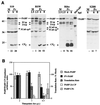
Poliovirus (PV) causes a rapid and drastic inhibition of host cell cap-dependent protein synthesis during infection while preferentially allowing cap-independent translation of its own genomic RNA via an internal ribosome entry site element. Inhibition of cap-dependent translation is partly mediated by cleavage of an essential translation initiation factor, eIF4GI, during PV infection. In addition to cleavage of eIF4GI, cleavage of eIF4GII and poly(A)-binding protein (PABP) has been recently proposed to contribute to complete host translation shutoff; however, the relative importance of eIF4GII and PABP cleavage has not been determined. At times when cap-dependent translation is first blocked during infection, only 25 to 35% of the total cellular PABP is cleaved; therefore, we hypothesized that the pool of PABP associated with polysomes may be preferentially targeted by viral proteases. We have investigated what cleavage products of PABP are produced in vivo and the substrate determinants for cleavage of PABP by 2A protease (2A(pro)) or 3C protease (3C(pro)). Our results show that PABP in ribosome-enriched fractions is preferentially cleaved in vitro and in vivo compared to PABP in other fractions. Furthermore, we have identified four N-terminal PABP cleavage products produced during PV infection and have shown that viral 3C protease generates three of the four cleavage products. Also, 3C(pro) is more efficient in cleaving PABP in ribosome-enriched fractions than 2A(pro) in vitro. In addition, binding of PABP to poly(A) RNA stimulates 3C(pro)-mediated cleavage and inhibits 2A(pro)-mediated cleavage. These results suggest that 3C(pro) plays a major role in processing PABP during virus infection and that the interaction of PABP with translation initiation factors, ribosomes, or poly(A) RNA may promote its cleavage by viral 2A and 3C proteases.
Figures
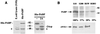
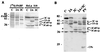





Similar articles
-
Cleavage of poly(A)-binding protein by poliovirus 3C protease inhibits host cell translation: a novel mechanism for host translation shutoff.Mol Cell Biol. 2004 Feb;24(4):1779-90. doi: 10.1128/MCB.24.4.1779-1790.2004. Mol Cell Biol. 2004. PMID: 14749392 Free PMC article.
-
Modulation of enteroviral proteinase cleavage of poly(A)-binding protein (PABP) by conformation and PABP-associated factors.Virology. 2008 May 25;375(1):59-72. doi: 10.1016/j.virol.2008.02.002. Epub 2008 Mar 5. Virology. 2008. PMID: 18321554 Free PMC article.
-
Cleavage of poly(A)-binding protein by poliovirus 3C proteinase inhibits viral internal ribosome entry site-mediated translation.J Virol. 2008 Oct;82(19):9389-99. doi: 10.1128/JVI.00006-08. Epub 2008 Jul 16. J Virol. 2008. PMID: 18632855 Free PMC article.
-
The interaction of cytoplasmic RNA viruses with the nucleus.Virus Res. 2003 Sep;95(1-2):75-85. doi: 10.1016/s0168-1702(03)00164-3. Virus Res. 2003. PMID: 12921997 Review.
-
Picornavirus 3C Proteins Intervene in Host Cell Processes through Proteolysis and Interactions with RNA.Viruses. 2023 Dec 12;15(12):2413. doi: 10.3390/v15122413. Viruses. 2023. PMID: 38140654 Free PMC article. Review.
Cited by
-
Enterovirus Control of Translation and RNA Granule Stress Responses.Viruses. 2016 Mar 30;8(4):93. doi: 10.3390/v8040093. Viruses. 2016. PMID: 27043612 Free PMC article. Review.
-
Viral modulation of stress granules.Virus Res. 2012 Nov;169(2):430-7. doi: 10.1016/j.virusres.2012.06.004. Epub 2012 Jun 15. Virus Res. 2012. PMID: 22705970 Free PMC article. Review.
-
Seneca Valley Virus 3Cpro Cleaves PABPC1 to Promote Viral Replication.Pathogens. 2020 Jun 4;9(6):443. doi: 10.3390/pathogens9060443. Pathogens. 2020. PMID: 32512928 Free PMC article.
-
Cleavage of poly(A)-binding protein by duck hepatitis A virus 3C protease.Sci Rep. 2017 Nov 24;7(1):16261. doi: 10.1038/s41598-017-16484-1. Sci Rep. 2017. PMID: 29176600 Free PMC article.
-
Poliovirus replication requires the N-terminus but not the catalytic Sec7 domain of ArfGEF GBF1.Cell Microbiol. 2010 Oct;12(10):1463-79. doi: 10.1111/j.1462-5822.2010.01482.x. Cell Microbiol. 2010. PMID: 20497182 Free PMC article.
References
-
- Asselbergs, F., W. Peters, W. van Venrooij, and H. Bloemendal. 1978. Diminished sensitivity of re-initiation of translation to inhibition by cap analogues in reticulocyte lysates. Eur. J. Biochem. 88:483-488. - PubMed
-
- Bag, J., and J. Wu. 1996. Translational control of poly(A)-binding protein expression. Eur. J. Biochem. 237:143-152. - PubMed
-
- Baker, E. J. 1993. Control of poly(A) length, p. 367-415. In J. G. Belasco and G. Brawerman (ed.), Control of messenger RNA stability. Academic Press, San Diego, Calif.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

