Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies
- PMID: 11836389
- PMCID: PMC153825
- DOI: 10.1128/jvi.76.5.2123-2130.2002
Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies
Abstract
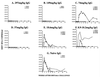
We previously reported that high-titered neutralizing antibodies directed against the human immunodeficiency virus type 1 (HIV-1) envelope can block the establishment of a simian immunodeficiency virus (SIV)/HIV chimeric virus (SHIV) infection in two monkeys following passive transfer (R. Shibata et al., Nat. Med. 5:204-210, 1999). In the present study, increasing amounts of neutralizing immunoglobulin G (IgG) were administered to 15 pig-tailed macaques in order to obtain a statistically valid protective neutralization endpoint titer in plasma. Using an in vitro assay which measures complete neutralization of the challenge SHIV, we correlated the titers of neutralizing antibodies in plasma at the time of virus inoculation (which ranged from 1:3 to 1:123) with the establishment of infection in virus-challenged animals. Ten of 15 monkeys in the present experiment were virus free as a result of neutralizing IgG administration as monitored by DNA PCR (peripheral blood mononuclear cells and lymph node cells), RNA PCR (plasma), virus isolation, and the transfer of lymph node cell suspensions (10(8) cells) plus 8 ml of whole blood from protected animals to naïve macaques. The titer of neutralizing antibodies in the plasma calculated to protect 99% of virus-challenged monkeys was 1:38.
Figures



References
-
- American Academy of Pediatrics. 1997. Report of the Committee on Infectious Diseases, 24th ed. American Academy of Pediatrics, Elk Grove Village, Ill.
-
- Brander, C., and B. D. Walker. 1999. T lymphocyte responses in HIV-1 infection: implications for vaccine development. Curr. Opin. Immunol. 11:451-459. - PubMed
-
- Cho, M. W., Y. B. Kim, M. K. Lee, K. C. Gupta, W. Ross, R. Plishka, A. Buckler-White, T. Igarashi, T. Theodore, R. Byrum, C. Kemp, D. C. Montefiori, and M. A. Martin. 2001. Polyvalent envelope glycoprotein vaccine elicits a broader neutralizing antibody response but is unable to provide sterilizing protection against heterologous simian/human immunodeficiency virus infection in pigtailed macaques. J. Virol. 75:2224-2234. - PMC - PubMed
-
- Committee on Care and Use of Laboratory Animals. 1985. Guide for the care and use of laboratory animals. NIH 85-23. Department of Health and Human Services, Washington, D.C.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

