Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment
- PMID: 11836412
- PMCID: PMC153830
- DOI: 10.1128/jvi.76.5.2340-2349.2002
Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment
Abstract
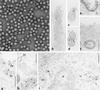
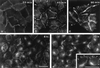
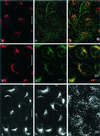
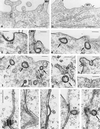
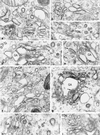
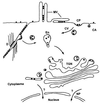
Among the adeno-associated virus (AAV) serotypes which are discussed as vectors for gene therapy AAV type 5 (AAV5) represents a candidate with unique advantages. To further our knowledge on AAV5-specific characteristics, we studied the entry pathway of wild-type virus in HeLa cells in the absence of helper virus by immunofluorescence and electron microscopy and by Western blot analysis. We found virus binding at the apical cell surface, especially at microvilli and, with increasing incubation time, virus accumulation at cell-cell boundaries. The different binding kinetics suggest different binding properties at apical versus lateral plasma membranes. Endocytosis of viruses was predominantly by clathrin-coated vesicles from both membrane domains; however, particles were also detected in noncoated pits. AAV5 particles were mainly routed to the Golgi area, where they could be detected within cisternae of the trans-Golgi network and within vesicles associated with cisternae and with the dictyosomal stacks of the Golgi apparatus. These data suggest that AAV5 makes use of endocytic routes that have hitherto not been described as pathways for virus entry.
Figures

References
-
- Bantel-Schaal, U., and H. zur Hausen. 1984. Characterization of the DNA of a defective parvovirus isolated from a genital site. Virology 134:52-63. - PubMed
-
- Bantel-Schaal, U., and H. zur Hausen. 1988. Adeno-associated viruses inhibit SV40 DNA amplification and replication of herpes simplex virus in SV40-transformed hamster cells. Virology 164:64-74. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

