Epstein-Barr virus BALF1 is a BCL-2-like antagonist of the herpesvirus antiapoptotic BCL-2 proteins
- PMID: 11836425
- PMCID: PMC153809
- DOI: 10.1128/jvi.76.5.2469-2479.2002
Epstein-Barr virus BALF1 is a BCL-2-like antagonist of the herpesvirus antiapoptotic BCL-2 proteins
Abstract
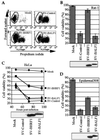
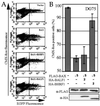
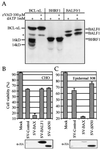
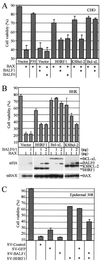
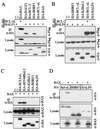
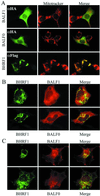
Cellular BCL-2 family proteins can inhibit or induce programmed cell death in part by counteracting the activity of other BCL-2 family members. All sequenced gammaherpesviruses encode a BCL-2 homologue that potently inhibits apoptosis and apparently escapes some of the regulatory mechanisms that govern the functions of their cellular counterparts. Examples of these protective proteins include BHRF1 of Epstein-Barr virus (EBV) and KSBcl-2 of Kaposi's sarcoma-associated herpesvirus, also known as human herpesvirus 8. The gamma-1 subgroup of these viruses, such as EBV, encodes a second BCL-2 homologue. We have now found that this second BCL-2 homologue encoded by EBV, BALF1, inhibits the antiapoptotic activity of EBV BHRF1 and of KSBcl-2 in several transfected cell lines. However, BALF1 failed to inhibit the cellular BCL-2 family member, BCL-x(L). Thus, BALF1 acts as a negative regulator of the survival function of BHRF1, similar to the counterbalance observed between cellular BCL-2 family members. Unlike the cellular BCL-2 family antagonists, BALF1 lacked proapoptotic activity and could not be converted into a proapoptotic factor in a manner similar to cellular BCL-2 proteins by caspase cleavage or truncation of the N terminus. Coimmunoprecipitation experiments and immunofluorescence assays suggest that a minimal amount, if any, of the BHRF1 and BALF1 proteins colocalizes inside cells, suggesting that mechanisms other than direct interaction explain the suppressive function of BALF1.
Figures

References
-
- Adams, J. M., and S. Cory. 1998. The Bcl-2 protein family: arbiters of cell survival. Science 281:1322-1326. - PubMed
-
- Basanez, G., A. Nechushtan, O. Drozhinin, A. Chanturiya, E. Choe, S. Tutt, K. A. Wood, Y.-T. Hsu, J. Zimmerberg, and R. J. Youle. 1999. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc. Natl. Acad. Sci. USA 96:5492-5497. - PMC - PubMed
-
- Basanez, G., J. Zhang, B. N. Chau, G. I. Maksaev, V. Frolov, T. A. Brandt, J. Burch, J. M. Hardwick, and J. Zimmerberg. 2001. Pro-apoptotic cleavage products of Bcl-xL form cytochrome c-conducting pores in pure lipid bilayers. J. Biol. Chem. 276:31083-31091. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

