Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence
- PMID: 11836430
- PMCID: PMC153797
- DOI: 10.1128/jvi.76.5.2518-2528.2002
Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence
Abstract
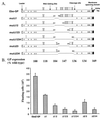
The Ebola virus envelope glycoprotein (GP) derived from the pathogenic Zaire subtype mediates cell rounding and detachment from the extracellular matrix in 293T cells. In this study we provide evidence that GPs from the other pathogenic subtypes, Sudan and Côte d'Ivoire, as well as from Reston, a strain thought to be nonpathogenic in humans, also induced cell rounding, albeit at lower levels than Zaire GP. Sequential removal of regions of potential O-linked glycosylation at the C terminus of GP1 led to a step-wise reduction in cell detachment without obviously affecting GP function, suggesting that such modifications are involved in inducing the detachment phenotype. While causing cell rounding and detachment in 293T cells, Ebola virus GP did not cause an increase in cell death. Indeed, following transient expression of GP, cells were able to readhere and continue to divide. Also, the rounding effect was not limited to 293T cells. Replication-deficient adenovirus vectors expressing Ebola virus GP induced the loss of cell adhesion in a range of cell lines and primary cell types, including those with proposed relevance to Ebola virus infection in vivo, such as endothelial cells and macrophages. In both transfected 293T and adenovirus-infected Vero cells, a reduction in cell surface expression of adhesion molecules such as integrin beta1 concurrent with the loss of cell adhesion was observed. A number of other cell surface molecules, however, including major histocompatibility complex class I and the epidermal growth factor receptor, were also down-modulated, suggesting a global mechanism for surface molecule down-regulation.
Figures






References
-
- Anonymous. 1990. Update: filovirus infection in animal handlers. Morbid. Mortal. Wkly. Rep. 39:221. - PubMed
-
- Baize, S., E. M. Leroy, M. C. Georges-Courbot, M. Capron, J. Lansoud-Soukate, P. Debre, S. P. Fisher-Hoch, J. B. McCormick, and A. J. Georges. 1999. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 5:423-426. - PubMed
-
- Balter, M. 2000. On the trail of Ebola and Marburg viruses. Science 290:923-924. - PubMed
-
- Baskerville, A., S. P. Fisher-Hoch, G. H. Neild, and A. B. Dowsett. 1985. Ultrastructural pathology of experimental Ebola haemorrhagic fever virus infection. J. Pathol. 147:199-209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

